Topical Gene Therapy FDA-Approved for Severe Skin Disease, Dystrophic Epidermolysis Bullosa
PLOS: DNA Science
JUNE 1, 2023
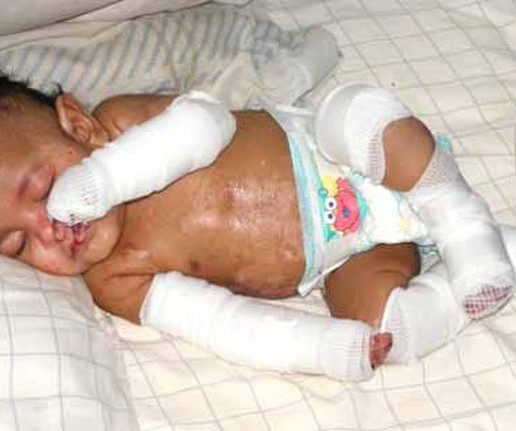
DEB has been a candidate for a gene therapy since 2002. “Until now, doctors and nurses had no way to stop blisters and wounds from developing on dystrophic EB patient skin and all we could do was to give them bandages and helplessly watch as new blisters formed. RDEB is inherited from two carrier parents.









Let's personalize your content