Drug Channels News Roundup, February 2021: Accumulator Problems, Humana + ESI, White Bagging Battles, Buy-and-Bill Economics, and Pharmacy Hero Dave Marley
Drug Channels
FEBRUARY 23, 2021
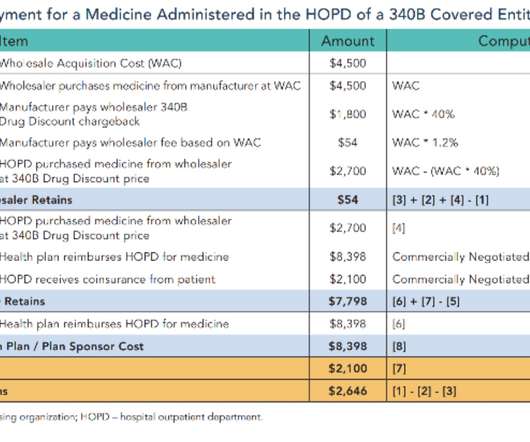
In this issue: Payers confess: Patients lose from copay accumulators Humana joins the Express Scripts GPO Hospitals vs. PBMs over specialty pharmacy white bagging Let’s all follow the Buy-and-Bill Dollar! Plus, entrepreneurial pharmacy owner Dave Marley demonstrates the power of innovation. Sometimes, the little guy wins big.













Let's personalize your content