CAR-NK cells: promising for cancer therapy
Drug Target Review
NOVEMBER 17, 2023
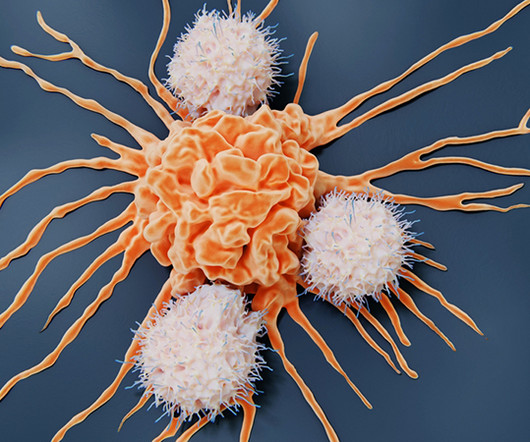
Up until 2023 around 600 NK cell clinical trials have been reported and some 200 are clinically active and still recruiting patients. The vast majority (65/74; 88%) of those trials involved NK cells without CARs; while only 12 percent were CAR-NK studies. Only three of the CAR-NK studies were for the treatment of solid tumours.




































Let's personalize your content