Seladelpar
New Drug Approvals
SEPTEMBER 8, 2024
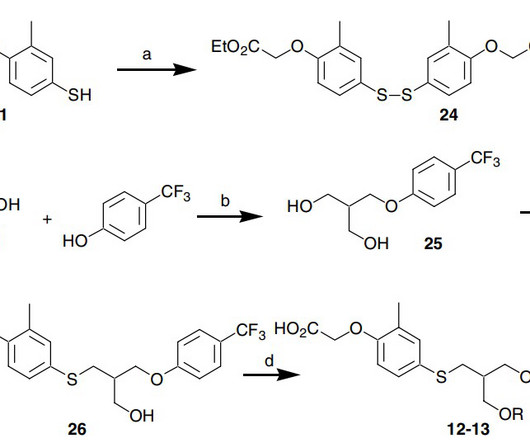
4] Seladelpar was approved for medical use in the United States in August 2024. [1] 2007.05.007 Drug Discovery, Johnson and Johnson Pharmaceutical Research and Development, LLC, 8 Clarke Drive, Cranbury, NJ 08512, USA Scheme 1. FDA” (Press release). Seladelpar cas 851528-79-5 C 21 H 23 F 3 O 5 S, 444.47 2007.05.007.














Let's personalize your content