FDA Approvals Roundup: Kloxxado, Farxiga, Ferriprox
The Pharma Data
MAY 5, 2021
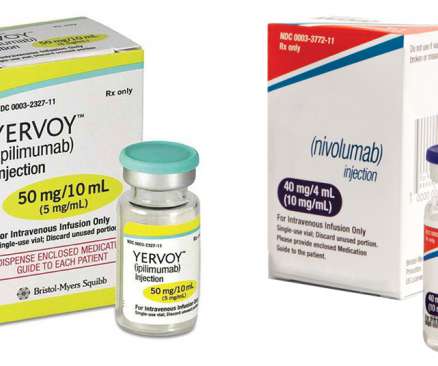
Farxiga use expanded for reducing risks in patients with chronic kidney disease. Approval of the sodium-glucose cotransporter 2 (SGLT2) inhibitor was based on efficacy findings in the interventional, multicenter, randomized Dapa-CKD study. controlled trial had not received previous systemic therapy for metastatic disease.































Let's personalize your content