Advancing CAR-T therapy: how CD5 modulation is shaping cancer treatment
Drug Target Review
AUGUST 29, 2024
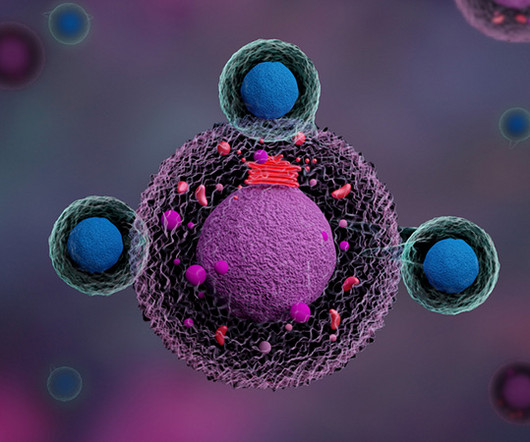
MR : Chimeric antigen receptor T-cell (CAR-T) therapy is very effective in treating patients with B-cell lymphoma, leukemia, and multiple myeloma, where we have six FDA-approved drugs. However, these treatments will eventually fail for the majority of patients, so there is a strong need for better CAR therapies.





































Let's personalize your content