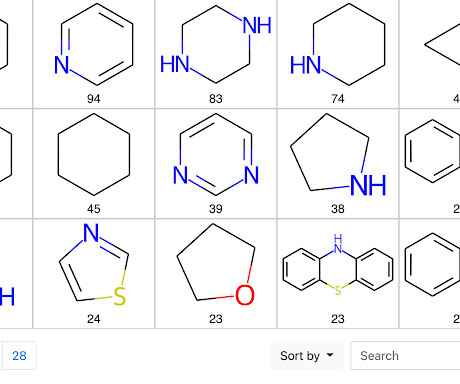
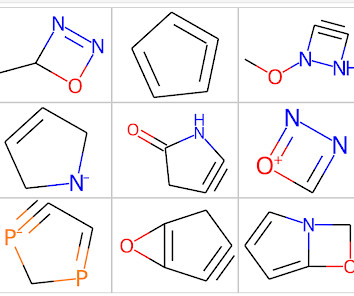
Mining Ring Systems in Molecules for Fun and Profit
Practical Cheminformatics
DECEMBER 4, 2022
Finally, we will look at a simple application of the method and explore the ring systems in marketed drugs. In addition, the core code for extracting ring systems from molecules has been incorporated into the latest version of my pip installable useful_rdkit_utils package. Mühlbacher, J., Schuffenhauer, A., & & Selzer, P.




































Let's personalize your content