Viral Vectors 101: What is a Virus (and a Viral Vector)?
addgene Blog
AUGUST 18, 2023
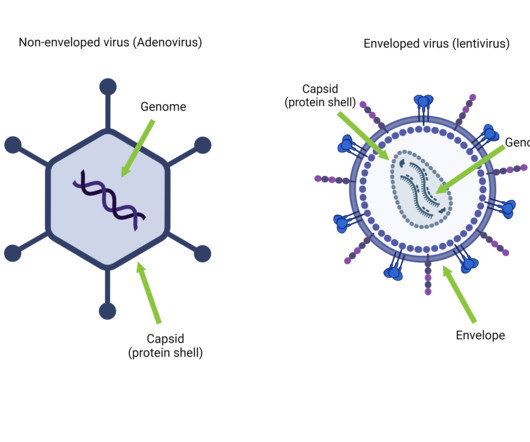
Viruses are simple – a genome packaged in a protein shell (Taylor, 2014). They’re so simple that we can’t even decide if they’re alive or not. Yet these simple, small particles have quite the outsized impact – and not just on the disease front.









































Let's personalize your content