Antibody Drug Conjugates: windows of opportunity
Drug Target Review
JULY 4, 2023
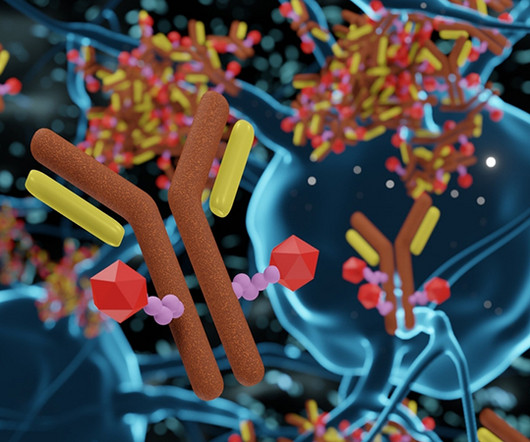
These therapies have broadened treatment options for patients to expand beyond the more traditional small molecule drug alternatives. 2 However, when dosed at the MTD, ADCs display improved efficacy over small molecules in oncology trials. 3D rendering of Antibody Drug Conjugate Molecules.









Let's personalize your content