The genetic modifier approach: identifying the right target for rare diseases
Drug Target Review
JUNE 21, 2023
Most rare diseases are caused by a single gene defect, but severity can vary considerably among patients. Modifier genes can help explain that variability and can alter or even prevent disease onset and progression, making them appealing therapeutic targets. However, the identification of these genes is challenging.



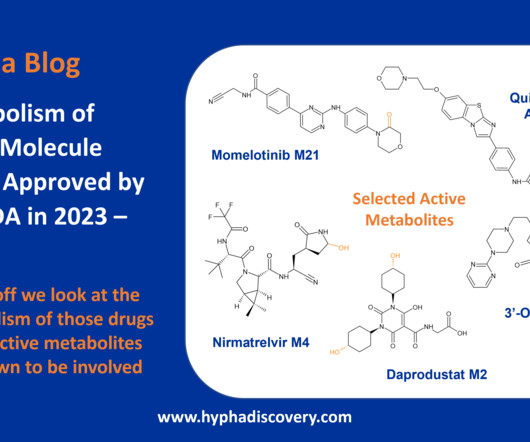






































Let's personalize your content