Metabolism of 2023 FDA Approved Small Molecules – PART 1
Metabolite Tales Blog
JANUARY 23, 2024
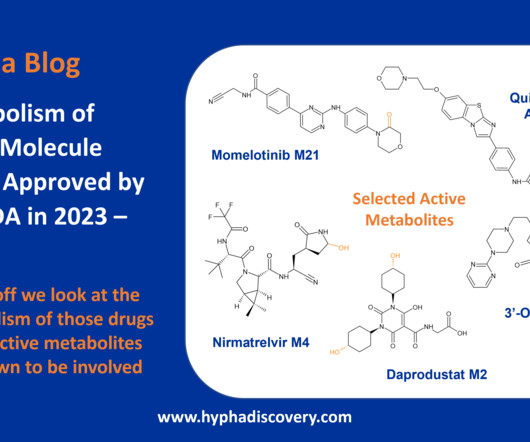
Metabolism of 2023 FDA Approved Small Molecules – PART 1 By Julia Shanu-Wilson 2023 was a fruitful year for drug approvals by the FDA, with a crop of 34 small molecules out of a total of 55 new drugs [1]. link] [3] FDA prescribing information for daprodustat. Pharmacokinetics and Metabolism of Nirmatrelvir.












Let's personalize your content