Have You Missed Any FDA Data Submission Requirements? A Look at Substantial Changes and New Requirements
Cytel
JANUARY 3, 2024
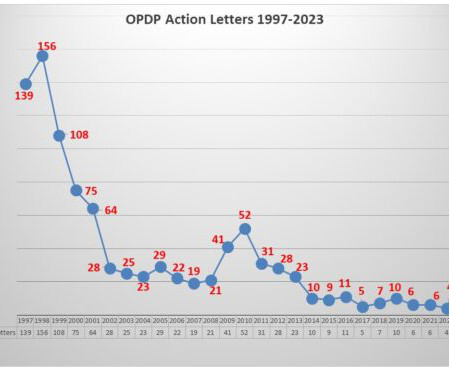
1 The following December, a second version was released after public comments and, on December 16, 2014, the FDA stopped the clock, providing sponsors with a pivotal two-year window to adapt their methods of creating clinical dataset packages to comply with the FDA’s new required data standards for any study commencing after December 16, 2016.











































Let's personalize your content