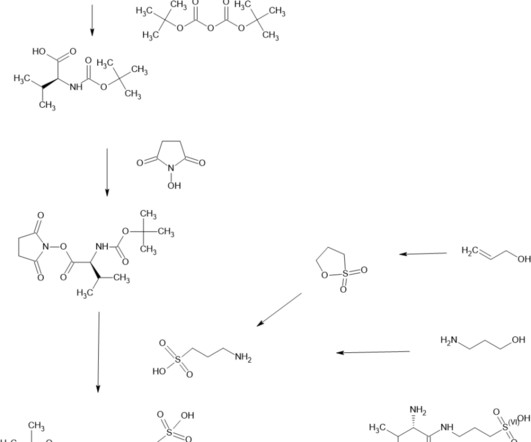
VALILTRAMIPROSATE
New Drug Approvals
OCTOBER 12, 2024
Alzheon licensed ALZ-801 from NeuroChem and is developing it for Alzheimer’s disease. ALZ-801 is an advanced and markedly improved candidate for the treatment of alzheimer’s disease. Clinical Pharmacokinetics and Safety of ALZ-801, a Novel Prodrug of Tramiprosate in Development for the Treatment of Alzheimer’s Disease.













































Let's personalize your content