Metabolism of 2022 FDA approved small molecule drugs PART 1
Metabolite Tales Blog
JANUARY 26, 2023
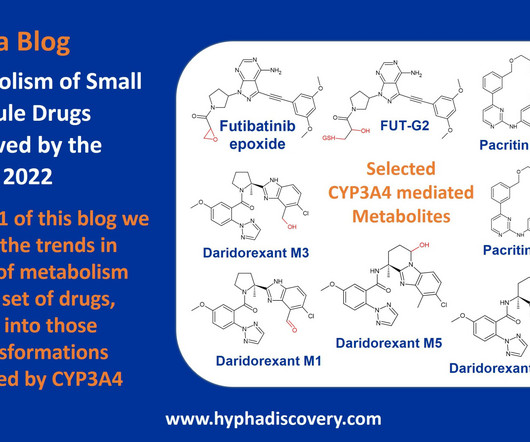
Metabolism of 2022 FDA approved small molecule drugs – Part 1 Does CYP3A4 still rule? By Julia Shanu-Wilson It won’t come as much surprise to learn that of the 17 small molecules* approved by the FDA in 2022, CYP3A4 was the major player in drug metabolism. Sugano, 2018.



















Let's personalize your content