GSK antibody drug reduces COPD attacks in trial
BioPharma Drive: Drug Pricing
SEPTEMBER 6, 2024
regulators rejected GSK’s submission in 2018. New trial results could offer support for an expansion of Nucala’s label after U.S.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
SEPTEMBER 6, 2024
regulators rejected GSK’s submission in 2018. New trial results could offer support for an expansion of Nucala’s label after U.S.

Drugs.com
OCTOBER 1, 2024
government trials did between 2018 and 2022, new research shows.The study -- conducted by researchers at Fred Hutch Cancer Center in. TUESDAY, Oct. 1, 2024 -- Clinical trials sponsored by Big Pharma enrolled eight times as many patients as U.S.-government
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drugs.com
SEPTEMBER 7, 2023
7, 2023 -- Emergency room visits for injuries related to driving under the influence of cannabis skyrocketed in Canada after the drug was legalized there, a new study reports. In October 2018, Canada became the second country to. THURSDAY, Sept.

Drugs.com
DECEMBER 4, 2024
4, 2024 -- About a third of America’s pharmacies have closed since 2010, amounting to an “unprecedented decline” in neighborhood drug stores, a new study finds.The drop began in 2018, primarily driven by store closures among chain ph. WEDNESDAY, Dec.

Drug Patent Watch
DECEMBER 10, 2024
India has long been recognized as a significant player in the global pharmaceutical industry, particularly in the production of generic drugs. This article delves into India’s growing importance in generic drug API manufacturing, highlighting the key factors contributing to its success and the challenges it faces. Califf, M.D.,

BioPharma Drive: Drug Pricing
OCTOBER 22, 2024
A medicine the pharma acquired in a $430 million buyout of Visterra in 2018 succeeded in a Phase 3 trial in IgA nephropathy, a crowded corner of drug research.

BioPharma Drive: Drug Pricing
JANUARY 2, 2025
The agency’s main drug review office cleared 50 novel medicines last year, short of 2018’s record total but on the higher end of recent annual tallies.

BioPharma Drive: Drug Pricing
MAY 22, 2024
The deal, only Biogen’s third since the start of 2018, is a sign the company is open to branching out beyond the neurological drugs that have long been its focus.

BioPharma Drive: Drug Pricing
OCTOBER 4, 2024
Having already been on a winding journey, Enjaymo, which Sanofi acquired through a 2018 buyout of Bioverativ, is now headed to Recordati as part of a deal announced Friday.

BioPharma Drive: Drug Pricing
MARCH 17, 2024
The biotech, formed as a successor to a company Roche acquired in 2018, has two drugs in clinical testing for neurological and immune diseases.
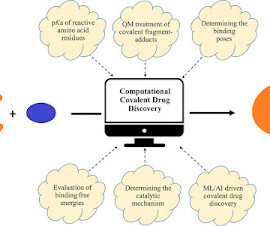
Covalent Modifiers
FEBRUARY 6, 2024
3c04710 Covalent drug discovery has been a challenging research area given the struggle of finding a sweet balance between selectivity and reactivity for these drugs, the lack of which often leads to off-target activities and hence undesirable side effects. Recent developments in in silico methods provide promise in this direction.
Crown Bioscience
JUNE 6, 2024
In a significant development, the US Food and Drug Administration’s (FDA) Center for Drug Evaluation and Research (CDER) approved 55 new drugs in 2023.

Drug Hunter
JUNE 5, 2023
Macrocyclic molecules, containing 12 or more atoms in a ring system, are increasingly prevalent in drug discovery, with over 67 macrocyclic drugs approved so far and several notable examples of clinical success highlighted on Drug Hunter recently, including MK-0616 , repotrectinib , and pacritinib.

Agency IQ
SEPTEMBER 22, 2023
Following a 2018 draft framework, FDA unveils long-awaited PDURS draft guidance A long-awaited draft guidance on Prescription Drug Use-Related Software (PDURS) outlines when information from digital health tools could be represented on prescription drug labeling. Fill out the form to read the full article.
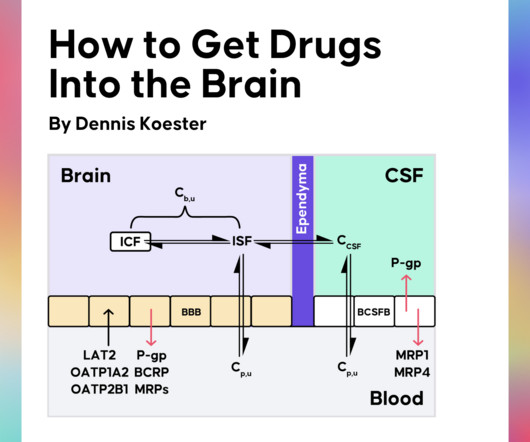
Drug Hunter
AUGUST 21, 2023
Obtaining adequate drug exposure in the brain is key to treating CNS diseases effectively. Recently, Dennis Koester gave us a crash course in CNS drug discovery in a Drug Hunter Flash Talk. Why Kp,uu is the Most Important Parameter in CNS Drug Discovery What Influences the Kp,uu of Drugs?

The Pharma Data
NOVEMBER 17, 2020
17, 2020 — From 2009 to 2018, there were increases in diseases-of-despair diagnoses, according to a study published online Nov. The prevalence of alcohol-related, substance-related, and suicide-related diagnoses increased by 37, 94, and 170 percent, respectively, from 2009 to 2018. TUESDAY, Nov. 9 in BM J Open. © 2020 HealthDay.

Drug Target Review
SEPTEMBER 26, 2023
These cells demonstrate considerable promise for uncovering drug-induced perturbations to neuronal function such as seizure, and their use extends further to sedation, anti-epileptic drug discovery and modelling of neurological diseases.

Broad Institute
JANUARY 22, 2024
At the Broad Institute of MIT and Harvard and elsewhere, researchers are also learning how dependencies affect cancer cells and how they influence each other and contribute to drug resistance. You’d need to disable both genes to see how important they are as a drug target. We miss that combinatorial dependence in our current screens.

Advarra
JULY 26, 2022
However, it’s not legal federally and is considered a Schedule I drug by the U.S. Drug Enforcement Administration (DEA), meaning it has no accepted medical use and a high potential for abuse. The 2018 Farm Bill. The 2018 Farm Bill removed hemp from the Controlled Substance Act. Funding Research on Cannabis.

Drug Target Review
SEPTEMBER 20, 2023
“AI will not replace drug discovery scientists, but drug discovery scientists who use AI will replace those who don’t” – comment during EFMC meeting 2018 Progressing a drug molecule from concept to commercialisation typically takes 10-15 years and has high associated costs of up to $2 billion per launched drug, if all failures are factored in.

Antidote
JULY 29, 2022
The process of getting a new drug to market is an expensive one. Between 2009 and 2018, U.S. biopharmaceutical companies spent about $1 billion per drug according to an analysis published in JAMA , and other studies have found that it can cost up to $2.8 billion to bring a new therapy to market.

Drug Channels
JULY 6, 2022
The boom in copay accumulators and maximizers has radically shifted payers’ views on manufacturers’ copay support programs for specialty drugs. Below, I review a new survey of commercial plan sponsors and contrast the findings to a comparable survey from 2018. d/b/a Drug Channels Institute. to 1:30 p.m.
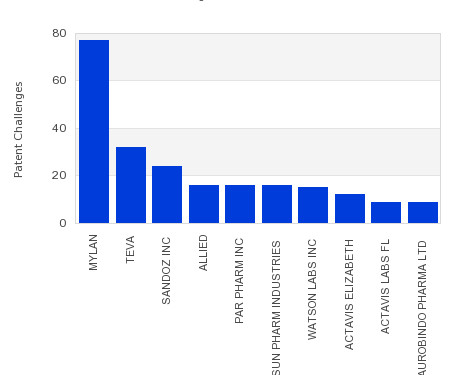
Drug Patent Watch
NOVEMBER 9, 2023
This chart shows the generic pharmaceutical companies that had the most successful drug patent challenges from 2018 to 2023. Companies that successfully challenge patents on branded drugs are granted six… The post Most prolific drug patent challengers appeared first on DrugPatentWatch - Make Better Decisions.

Agency IQ
SEPTEMBER 22, 2023
Following a 2018 draft framework, FDA unveils long-awaited PDURS draft guidance A long-awaited draft guidance on Prescription Drug Use-Related Software (PDURS) outlines when information from digital health tools could be represented on prescription drug labeling.
Drug Target Review
JULY 13, 2023
Drug discovery is a complex and vital field that continually seeks to identify new therapeutic targets and develop effective treatments. In recent years, a novel approach known as condensate biology has emerged, revolutionising the way researchers think about drug discovery and development.

FDA Law Blog: Drug Discovery
JULY 17, 2024
An Opportunity to Leverage the Newly Created Rare Disease Advisory Committee across CDER and CBER In Frank and James’ 2018 proposal there was also a recommendation for the formation of a Rare Disease Advisory Committee, which would allow FDA access to experts in the science of small trials and other aspects of rare disease research.

PPD
SEPTEMBER 6, 2023
Mixing of service models — a strategy that drug developers are leveraging now more than ever — can bring life-changing therapies to market faster. In 2018, market utilization of FSO models was at 72%, with usage of FSP models lagging at 28%. Growth of the FSP market is steadily increasing.
The Pharma Data
JANUARY 27, 2021
27, 2021 — The number of adverse drug reactions (ADRs) associated with hydroxychloroquine and chloroquine more than doubled in 2020 compared with 2018 and 2019, according to a research letter published online Jan. Food and Drug Administration Adverse Event Reporting System database from Jan. 1, 2018, to Sept.
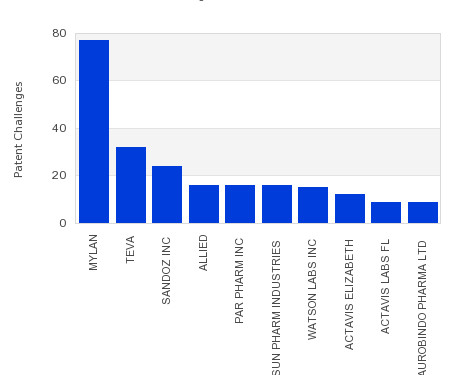
Drug Patent Watch
JULY 26, 2023
This chart shows the generic pharmaceutical companies that had the most successful drug patent challenges from 2018 to 2023. Companies that successfully challenge patents on branded drugs are granted six… The post Most prolific drug patent challengers appeared first on DrugPatentWatch - Make Better Decisions.
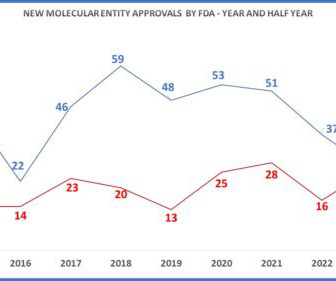
Eye on FDA
AUGUST 27, 2023
But in fact, as can be seen in previous years such as 2019, 2018 and 2015, the mid-year tally (in red) is not always predictive of what we may see by year’s end (in blue). Other Approvals – Of course, there are many drugs approved that are not NMEs and which do not have Advisory Committee meetings.

Common Sense for Drug Policy Blog
DECEMBER 14, 2023
Drugs and Social Media "End users seem to be buying their drugs on the dark web to a lesser extent than in previous years.28 28 Qualitative information provided by people who use social media suggests that the use of such media for drug purchasing purposes has been increasing, especially at the retail level.29

Elrig
MAY 8, 2024
How does your genetics affect your weight and can drugs help? Insights from Giles Yeo How does your genetics affect your weight and can drugs help? Originally GLP1-like drugs were developed to treat Type-2 diabetes as it also triggers insulin production. If you stop taking the drug, won’t the weight come back?

Drug Target Review
MARCH 3, 2025
The search for effective treatments for neurodegenerative diseases like Parkinson’s disease has long been hindered by the brain’s complexity and the absence of adequate models for drug discovery. “Then, the organoid model would be tested with, say, five, six, or seven drugs available on the market. .

Fierce BioTech
SEPTEMBER 13, 2024
Highlights From the Final ICH Drug Interactions Guideline pesurya Fri, 09/13/2024 - 11:39 Thu, 10/31/2024 - 11:00 Resource Type Webinar Brian Ogilvie Graham Dyck Duration 60 Minutes The ICH M12 Guideline was finalized on May 21st, 2024, after extensive review of industry and other comments.

Plenge Gen
JANUARY 6, 2019
At the 2018 Annual Atlas Ventures Retreat (AVR), I participated in a panel on Digital Health (along with David Schenkhein, John Reed, Scott Brun). While there are many aspects to digital health, we focused on the application to drug discovery and development. What is digital health in relation to drug discovery & development?

Drug Target Review
NOVEMBER 1, 2023
3 Another recent development is the implication of ATX in neurological diseases, 6,7 as ATX levels are related to metabolic disorders in Alzheimer disease, and thus might be an interesting biomarker and drug target for this devasting pathology. 2018 Jun 13;5. 2018 Sep;37(2–3):509–18. References: Ninou I, Magkrioti C, Aidinis V.
NIH Director's Blog: Drug Development
NOVEMBER 15, 2018
They’re synthesized in the lab, allowing Wimley and team to tweak their chemical structures and hopefully create ones with therapeutic potential, particularly as smart-delivery systems to target cells with greater precision and deliver biological cargoes such as drugs [1]. 2018 Jul 2;9(1):2568. [2] How can he tell the difference?
Drug Target Review
JANUARY 25, 2024
The peptide in development is based on a 2018 Cleveland Clinic discovery and serves as a proof-of-concept for this type of drug for triple-negative breast cancer. 2 The cells of this cancer lack certain receptors so drugs designed to treat other subtypes of breast cancer will not work.

Eye on FDA
JULY 28, 2020
By March we were remaining at home, including people who worked at FDA who assess new drugs for approval. Now after six months of the COVID-19 pandemic, one might have expected that the approval of new medicines by the Food and Drug Administration might have been negatively impacted as a result. But of course, that is what happened.

DrugBaron
FEBRUARY 16, 2021
More than a hundred antibodies have now been approved as drugs, making up around a third of all approvals over the last three years. Winter, together with George Smith and Frances Arnold, was also duly recognised with a Nobel Prize for this work in 2018. And many times, we don’t do a very good job even still.

Common Sense for Drug Policy Blog
AUGUST 22, 2023
Drug Checking Services "One of the consequences of drug prohibition is the lack of knowledge regarding the composition and purity of illicit substances ( Miron, 2003 ; Taylor et al., In the context of harm reduction, drug checking has emerged as a strategy to address this issue.

Common Sense for Drug Policy Blog
NOVEMBER 10, 2023
In 2017, the UN Committee on Economic, Social and Cultural Rights recommended that Russia change its punitive policy approach with an 18-month transformation and to consider decriminalising drugs for personal consumption. Drug control and human rights in the Russian Federation. But nothing has really changed. Nordisk Alkohol Nark.

The Pharma Data
MAY 5, 2021
FDA officials said that the number of a product specific guidances (PSGs) issued by the Office of Generic Drugs (OGD) has increased steadily since FY 2013. In FY 2019, 252 were issued, as were 208 in FY 2018. . FDA webinar on product specific guidance for generic drugs . Source link.
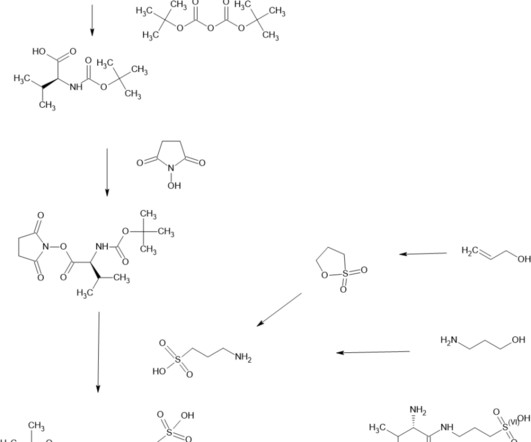
New Drug Approvals
OCTOBER 12, 2024
2018; 32(9): 849–861. [2]. 2018 Mar;57(3):315-333. Tramiprosate was reported to inhibit Aβ42 aggregation into toxic oligomers ( Gervais et al., 2007 ; Kocis et al., Both ALZ-801 and tramiprosate are metabolized to 3-sulfopranpanoic acid (3-SPA), which is normally found in brain and also inhibits Aβ42 aggregation ( Hey et al.,
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content