GSK antibody drug reduces COPD attacks in trial
BioPharma Drive: Drug Pricing
SEPTEMBER 6, 2024
New trial results could offer support for an expansion of Nucala’s label after U.S. regulators rejected GSK’s submission in 2018.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
SEPTEMBER 6, 2024
New trial results could offer support for an expansion of Nucala’s label after U.S. regulators rejected GSK’s submission in 2018.

Drugs.com
OCTOBER 1, 2024
1, 2024 -- Clinical trials sponsored by Big Pharma enrolled eight times as many patients as U.S.-government government trials did between 2018 and 2022, new research shows.The study -- conducted by researchers at Fred Hutch Cancer Center in. TUESDAY, Oct.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

BioPharma Drive: Drug Pricing
OCTOBER 22, 2024
A medicine the pharma acquired in a $430 million buyout of Visterra in 2018 succeeded in a Phase 3 trial in IgA nephropathy, a crowded corner of drug research.

Antidote
JULY 29, 2022
The process of getting a new drug to market is an expensive one. Between 2009 and 2018, U.S. biopharmaceutical companies spent about $1 billion per drug according to an analysis published in JAMA , and other studies have found that it can cost up to $2.8 billion to bring a new therapy to market.
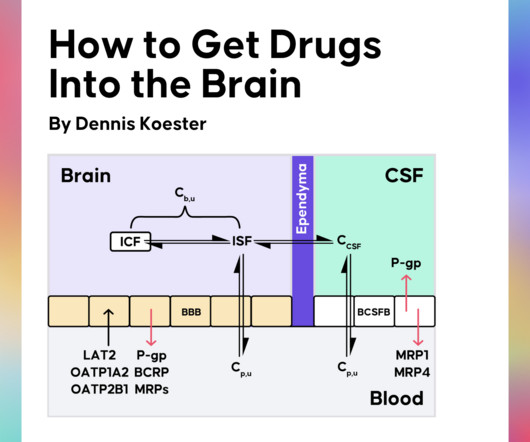
Drug Hunter
AUGUST 21, 2023
Obtaining adequate drug exposure in the brain is key to treating CNS diseases effectively. Recently, Dennis Koester gave us a crash course in CNS drug discovery in a Drug Hunter Flash Talk. Why Kp,uu is the Most Important Parameter in CNS Drug Discovery What Influences the Kp,uu of Drugs?

FDA Law Blog: Drug Discovery
JULY 17, 2024
An Opportunity to Leverage the Newly Created Rare Disease Advisory Committee across CDER and CBER In Frank and James’ 2018 proposal there was also a recommendation for the formation of a Rare Disease Advisory Committee, which would allow FDA access to experts in the science of small trials and other aspects of rare disease research.

PPD
SEPTEMBER 6, 2023
Approaches to outsourcing clinical trials have changed significantly in recent years. Mixing of service models — a strategy that drug developers are leveraging now more than ever — can bring life-changing therapies to market faster. Growth of the FSP market is steadily increasing. Overseen by an insourced project manager.

Drug Target Review
NOVEMBER 1, 2023
2 Implication of ATX in a large range of human diseases have been highlighted by both fundamental research and clinical trials. 1-5 Implication of ATX in a large range of human diseases have been highlighted by both fundamental research and clinical trials. 2018 Jun 13;5. References: Ninou I, Magkrioti C, Aidinis V. J Med Chem.

Drug Target Review
SEPTEMBER 26, 2023
These cells demonstrate considerable promise for uncovering drug-induced perturbations to neuronal function such as seizure, and their use extends further to sedation, anti-epileptic drug discovery and modelling of neurological diseases.

Vial
MAY 13, 2024
Introduction The biopharma industry is poised to make revolutionary advances in and redefine drug development, and the current climate for innovation appears ideal. However, progress from molecule to approved drug is hampered by extremely high costs and lengthy clinical trials , and approximately 90% of drugs that reach clinical trials fail.

Drug Target Review
SEPTEMBER 20, 2023
“AI will not replace drug discovery scientists, but drug discovery scientists who use AI will replace those who don’t” – comment during EFMC meeting 2018 Progressing a drug molecule from concept to commercialisation typically takes 10-15 years and has high associated costs of up to $2 billion per launched drug, if all failures are factored in.

Conversations in Drug Development Trends
FEBRUARY 28, 2023
Three months ago, he realized he knew little about the drug that saved his life, and he decided to look into how his chemotherapy was created. Rob found out that in 1980, a researcher in Birmingham, England first synthesized a very niche cancer drug in a small lab. But what was truly remarkable?
Drug Target Review
JANUARY 25, 2024
The peptide in development is based on a 2018 Cleveland Clinic discovery and serves as a proof-of-concept for this type of drug for triple-negative breast cancer. 2 The cells of this cancer lack certain receptors so drugs designed to treat other subtypes of breast cancer will not work.

New Drug Approvals
APRIL 25, 2025
12] However, absorption, metabolism, and excretion data of taselisib , a molecule with a related chemical scaffold, suggest moderately slow absorption into the systemic circulation, metabolism to play a minor role in drug clearance, and biliary excretion to be the main route of excretion. [13] Food and Drug Administration (FDA).

Agency IQ
SEPTEMBER 22, 2023
Following a 2018 draft framework, FDA unveils long-awaited PDURS draft guidance A long-awaited draft guidance on Prescription Drug Use-Related Software (PDURS) outlines when information from digital health tools could be represented on prescription drug labeling.

Drug Target Review
MARCH 3, 2025
The search for effective treatments for neurodegenerative diseases like Parkinson’s disease has long been hindered by the brain’s complexity and the absence of adequate models for drug discovery. This approach has the potential to revolutionise clinical trial design and lead to more effective, personalised treatments.

Drug Target Review
OCTOBER 11, 2023
One of our drug candidates at Samara activates Transient Receptor Potential Mucolipin 1 (TRPLM1), a protein that’s at the centre of the degradative machinery that’s so key to autophagy. Symptoms were reversed in mouse models and a clinical trial is planned for later this year. He holds an MBA from the University of Bath.

The Pharma Data
JANUARY 13, 2021
PITTSBURGH–( BUSINESS WIRE )– Knopp Biosciences LLC today announced positive top-line results in a Phase 2 dose-ranging trial of the novel oral drug dexpramipexole in patients with moderate-to-severe eosinophilic asthma. The trial was conducted at 28 U.S. 14, 2021 11:00 UTC. study centers. ABOUT THE EXHALE STUDY.

New Drug Approvals
APRIL 19, 2025
Mode of action The drug acts as an ultra-short-acting 1-selective blocking agent. Contrary to landiolol, exposure to other -blockers such as esmolol amplifies the re-expression of -receptors which explains the drug tolerance effect seen during long-term esmolol infusion. IV -Blocker max. 20 November 2023. 21 December 2022.
DrugBank
FEBRUARY 19, 2025
The application of Artificial Intelligence (AI) in drug discovery is rapidly transforming the pharmaceutical industry, offering opportunities to accelerate the identification of novel therapeutic targets, optimize molecule design, and enhance clinical trial efficiency. billion in 2023 to $7.9 billion in 2023 to $7.9
New Drug Approvals
OCTOBER 12, 2024
Years later, a subgroup analysis of the trial data indicated a potential positive effect in participants who carried two copies of ApoE4 ( Abushakra et al., 2018; 32(9): 849–861. [2]. 2018 Mar;57(3):315-333. After tramiprosate failed in Phase 3, its maker, NeuroChem, marketed it as a nutritional supplement. Hey JA, et al.

The Pharma Data
NOVEMBER 2, 2020
Novartis has announced that its approved migraine drug Aimovig (erenumab) has met its primary and secondary endpoints in a postmarket surveillance trial. Aimovig was approved by the FDA in May 2018 and received a marketing authorization from the European Medicines Agency in July 2018. Source link.

Drug Target Review
NOVEMBER 6, 2023
To bring us closer to curing cancer, a combination of effective drugs with non-overlapping mechanisms of action is required. 6 In all these examples, an effective backbone drug was first developed, before adding one or more drugs to establish the new regimens.

The Pharma Data
NOVEMBER 9, 2020
Trial Also Met the Primary Endpoint in Patients With Low Levels of Eosinophils. In the subgroup of patients with baseline eosinophil counts less than 300 cells per microliter, the trial met the primary endpoint with tezepelumab demonstrating a statistically significant and clinically meaningful reduction in AAER.

The Pharma Data
JANUARY 31, 2021
Adjuvanted S-Trimer COVID-19 vaccine candidates demonstrated favorable safety and tolerability profiles and strong neutralizing immune responses in a phase 1 trial. Clover plans to initiate a global phase 2/3 trial in the first half of 2021 with an interim analysis for vaccine efficacy potentially in the middle of 2021.

FDA Law Blog: Drug Discovery
DECEMBER 13, 2023
Drug development for these conditions has unique and complex challenges, therefore few treatments are available to patients.” However, there is tremendous potential in such a committee to advance rare disease drug development broadly. Note that FDA is currently soliciting applications to staff this committee.

Common Sense for Drug Policy Blog
AUGUST 22, 2023
Drug Checking Services and Image and Performance Enhancing Drugs "The Global Commission on Drug Policy recently advised governments to make harm reduction measures, including drug checking services, widely accessible ( Bewley-Taylor & Tinasti, 2020 ; Buxton et al., 2018 ; Olsen et al., 2018 ; Olsen et al.,

The Pharma Data
NOVEMBER 1, 2020
The trial is expected to enroll 160 patients. According to Frost & Sullivan analysis, global market of MS drugs reached US$23.0 billion in 2018, and it is expected to be up to US$48.9 MS is an autoimmune, inflammatory disease of the central nervous system. million people around the world are affected by MS today 1.
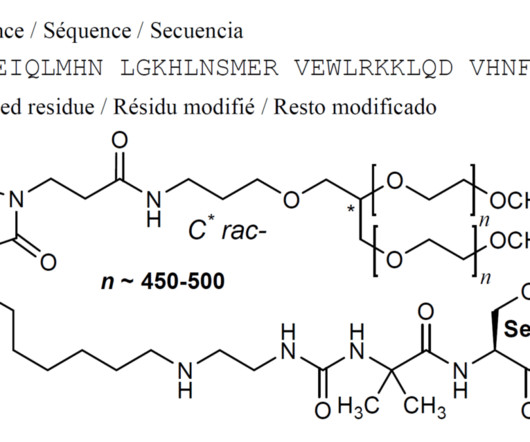
New Drug Approvals
SEPTEMBER 8, 2024
5] History The effectiveness of palopegteriparatide was evaluated in a 26-week, randomized, double-blind, placebo-controlled trial that enrolled 82 adults with hypoparathyroidism. [5] 5] Study drug and conventional therapy were subsequently adjusted according to the albumin-corrected serum calcium levels. [5] 14 August 2024.

Advarra
DECEMBER 14, 2023
Sponsor study team members and research sites each play a critical role in clinical trial executions. Sites working with an investigational new drug (IND) are often subject to an FDA site inspection. Despite the consequences, the FDA has reported a failure rate of 36% in clinical trial site inspections over the past five years.

The Pharma Data
DECEMBER 21, 2020
On Monday, the two companies said the investigational asset tezepelumab did not meet its primary endpoint in the Phase III SOURCE trial assessing the drug’s ability to generate a statistically significant reduction of corticosteroid use on top of standard of care treatment in some asthma patients. In 2018, the U.S.

Advarra
FEBRUARY 22, 2024
Assessing and reporting adverse events (AEs) in clinical trials is critical to ensuring the study is as safe as possible and the participants have the most up to date information so they can decide whether to continue their participation in the research study. Who Should Assess AEs in Clinical Trials? regulations.

Drug Target Review
FEBRUARY 28, 2024
Preclinical research on γδ T cells has made great strides since the cells were first identified in the 1980s, with γδ T-cell therapies from several companies, including IN8bio, now in or nearing clinical trials for various cancers. Trends Immunol 39(6):446-459 (2018). Nat Commun 9(1):1760 (2018). J Front Immunol 9:1409 (2018).
The Pharma Data
NOVEMBER 2, 2020
This morning, California-based GW said it will launch the first Phase III trial studying nabiximols, which is known as Sativex outside the United States, as a potential treatment for multiple sclerosis-associated spasticity. GW Pharmaceuticals hopes to bring its cannabis-based treatment for multiple sclerosis spasticity to the United States.

DrugBank
OCTOBER 4, 2024
For example, Gilead Sciences' acquisition of Immunomedics in 2020 included Trodelvy, an antibody-drug conjugate approved for treating triple-negative breast cancer. The IP surrounding Trodelvy , including patents covering the drug's composition, manufacturing process, and clinical use, was a significant valuation component.

The Pharma Data
DECEMBER 21, 2020
21, 2020 /PRNewswire/ — Amgen (NASDAQ:AMGN) and AstraZeneca today announced the SOURCE trial did not meet the primary endpoint of a statistically significant reduction in the daily oral corticosteroid (OCS) dose, without loss of asthma control, with tezepelumab compared to placebo. THOUSAND OAKS, Calif. ,

The Pharma Data
DECEMBER 31, 2020
Tabibiazar co-founded private Aravive Biologics and served as the Chairman of its board of directors and as President and Chief Executive Officer from its inception to April 2017 and as Executive Chairman from May 2017 until October 2018. since October 2018. to form the combined company, Aravive, Inc. ” Dr. .

The Pharma Data
JANUARY 6, 2021
7, 2021 /PRNewswire/ — UNION therapeutics A/S ( UNION ) today announces that the US Food and Drug Administration (FDA) has approved an Investigational New Drug program (IND) for oral orismilast; a next generation PDE4-inhibitor for the treatment of plaque psoriasis in adults. 2018)2: Menter MA, Armstrong AW, Gordon KB, Wu JJ.

The Pharma Data
DECEMBER 17, 2020
Opioid dependence is a serious, chronic, relapsing disease associated with a disproportionate amount of drug-related harm that includes infectious diseases and other health problems, mortality, unemployment, homelessness and social exclusion 6. Buvidal received market authorizations in EU and Australia in November 2018. About Camurus.

New Drug Approvals
SEPTEMBER 13, 2024
2] As of July 2022, it is in phase 3 clinical trials for major depressive disorder. [2] 10] However, a more recent study assessing neuroendocrine effects of the drug in normal volunteers and subjects with a history of cocaine dependence reported observations consistent with modest MOR antagonism at the 10 mg dose. [11] nM vs. 24.0

The Pharma Data
NOVEMBER 24, 2020
Food and Drug Administration announced Monday. Xofluza was approved in 2018 to treat uncomplicated flu in patients 12 years and older who are symptomatic for no more than two days. The drug was previously only available in a tablet form but is now available in granule form for mixing in water. TUESDAY, Nov.

Drug Target Review
OCTOBER 31, 2023
Regulatory affairs workers get to tell a story about a drug to the regulators and – keeping the end in mind, which is the drug label or prescribing information – is a good way to communicate with regulators. Every drug has a story: an end game. I’ve been lucky to have seen several drugs make it all the way to market.

The Pharma Data
JANUARY 14, 2021
Food and Drug Administration (FDA) approved Pfizer ’s Xalkori (crizotinib) for pediatric patients one year of age and older and young adults with relapsed or refractory, systemic anaplastic large cell lymphoma (ALCL) that is anaplastic lymphoma kinase (ALK)-positive. Manuel Esteban/Shutterstock.

Alta Sciences
OCTOBER 15, 2024
Up Close and Personal with Jason Boehme, Associate Director, Program Management nbartlett Tue, 10/15/2024 - 09:00 Jason Boehme joined Altasciences in 2018 as Senior Project Manager. Jason started his career as a research assistant carrying out operational phases of drug metabolism and pharmacokinetic studies. jpg Weight 1
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content