FDA Approved Oncology Drugs 2023
Crown Bioscience
JUNE 6, 2024
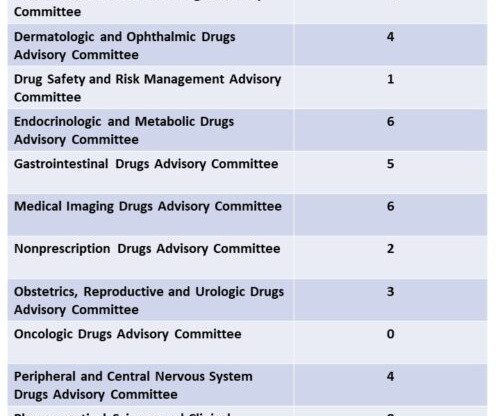
In a significant development, the US Food and Drug Administration’s (FDA) Center for Drug Evaluation and Research (CDER) approved 55 new drugs in 2023. This marks the second-highest count in the past 30 years, with the highest being 59 new drug approvals in 2018 and represents an impressive 50% increase in drugs approved in 2022.






































Let's personalize your content