Three trends in the antibody-drug conjugate (ADC) market
Drug Discovery World
JUNE 1, 2023
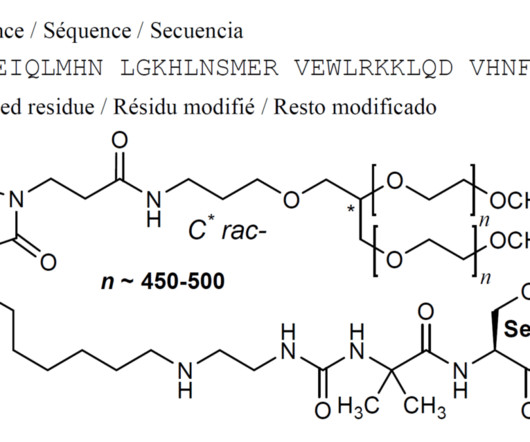
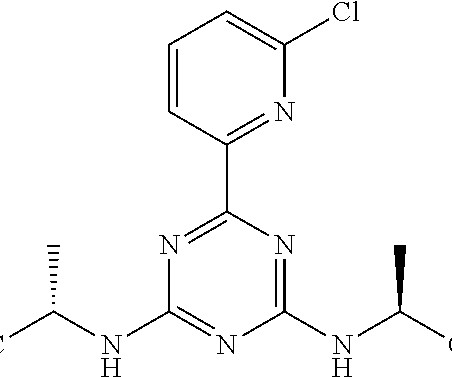
A Nature publication confirmed that there are currently 12 FDA-approved ADCs on the market, and nine of these secured FDA approval in the past six years 2. A market research report published in late June 2018 by HTF market Intelligence Consulting suggested that the ADC market in China would grow exponentially by 2023 4.





















Let's personalize your content