Advancing CAR-T therapy: how CD5 modulation is shaping cancer treatment
Drug Target Review
AUGUST 29, 2024
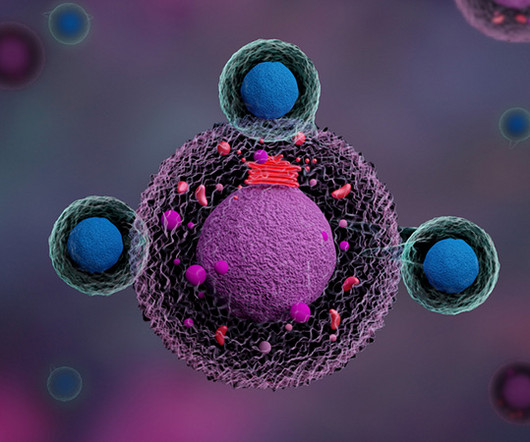
What potential advantages does the CD5 modulation strategy offer over traditional CAR-T therapies? MR : Chimeric antigen receptor T-cell (CAR-T) therapy is very effective in treating patients with B-cell lymphoma, leukemia, and multiple myeloma, where we have six FDA-approved drugs.









































Let's personalize your content