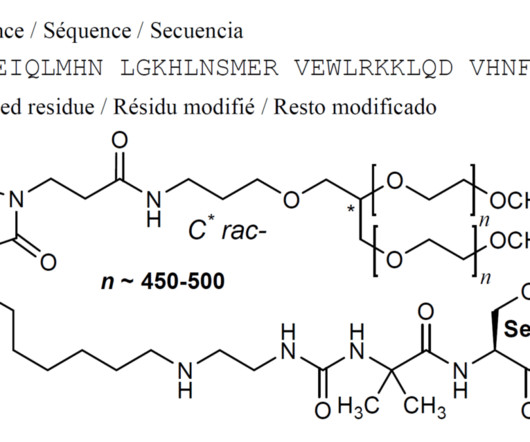
VALILTRAMIPROSATE
New Drug Approvals
OCTOBER 12, 2024
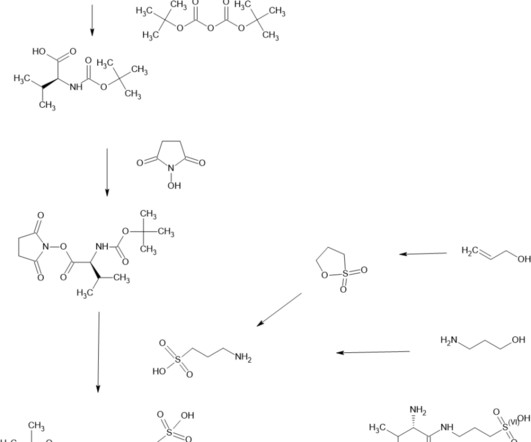
Alzheon licensed ALZ-801 from NeuroChem and is developing it for Alzheimer’s disease. ALZ-801 is an advanced and markedly improved candidate for the treatment of alzheimer’s disease. 2018; 32(9): 849–861. [2]. 2018 Mar;57(3):315-333. 2007 ; Kocis et al., 2016 ; Abushakra et al., Hey JA, et al. Clin Pharmacokinet.












































Let's personalize your content