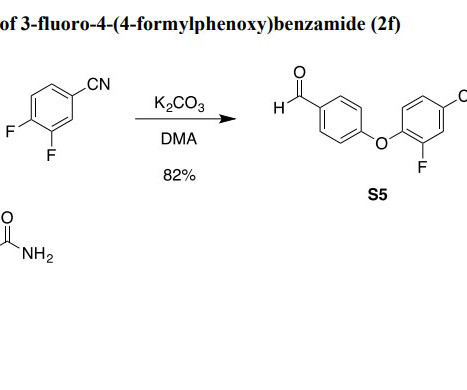
PureTech’s LYT-100 (Deupirfenidone) Demonstrates Tolerability and Pharmacokinetic Proof-of-Concept in Phase 1 Multiple Ascending Dose and Food Effect Study
The Pharma Data
NOVEMBER 17, 2020
The study demonstrated favorable proof-of-concept for LYT-100’s tolerability and pharmacokinetic (PK) profile, which will also enable twice-a-day (BID) dosing of LYT-100 in future studies. ERJ Open Research , 4(4), 00084-2018. doi:10.1183/23120541.00084-2018. 1 Cottin, V., Koschel, D., Günther, et al. Source link.






















Let's personalize your content