How MMR-deficient colorectal cancers regulate their growth
Drug Target Review
JULY 9, 2024
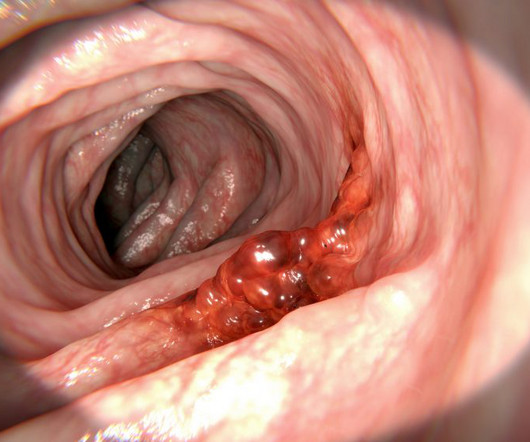
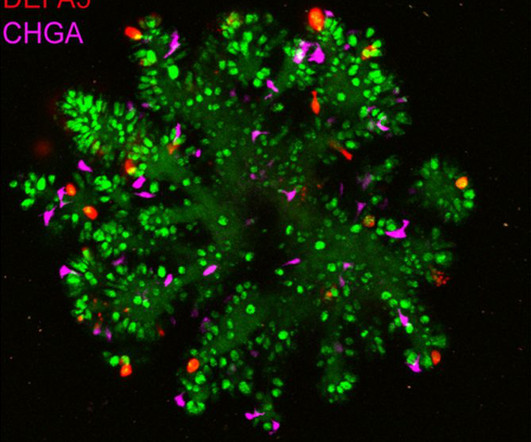
For the first time, scientists at UCL and University Medical Center Utrecht have observed bowel cancer cells’ ability to regulate their growth using a genetic on-off switch to increase their likelihood of survival. 2018 June 6 [cited 2024 July 8]; 8. Cancer Research UK. Cancer immunotherapy: broadening the scope of targetable tumours.













































Let's personalize your content