GSK antibody drug reduces COPD attacks in trial
BioPharma Drive: Drug Pricing
SEPTEMBER 6, 2024
regulators rejected GSK’s submission in 2018. New trial results could offer support for an expansion of Nucala’s label after U.S.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
SEPTEMBER 6, 2024
regulators rejected GSK’s submission in 2018. New trial results could offer support for an expansion of Nucala’s label after U.S.

Advarra
JULY 26, 2022
The 2018 Farm Bill. The 2018 Farm Bill removed hemp from the Controlled Substance Act. Importantly, the 2018 Farm Bill preserved FDA authority to regulate products with cannabis or cannabis-derived compounds under the Federal Food, Drug, and Cosmetic (FD&C) Act and Section 351 of the Public Health Service Act.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Agency IQ
AUGUST 25, 2023
Cannabis groups to Congress: FDA should regulate CBD as a dietary supplement U.S. legislators have asked for help to reimagine how the FDA should regulate cannabidiol (CBD) following the agency’s determination that it could not make use of its existing legislative or regulatory authorities to do so.
Drug Target Review
NOVEMBER 3, 2023
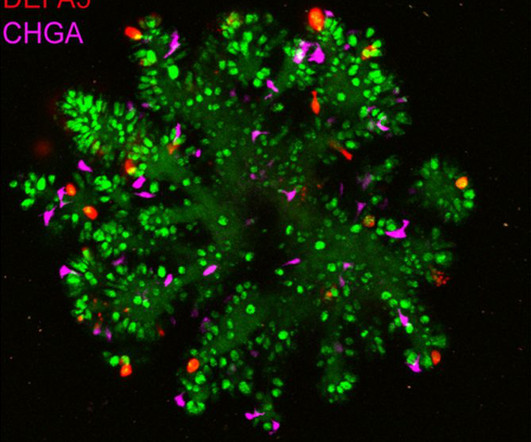
They identified ZNF800 as a key regulator of the differentiation of a specific gut cell type, the enteroendocrine cells. These hormones regulate digestive processes, like nutrient absorption, appetite, and glucose metabolism. 2018 February 8 [2023 November 1];172(4):650-65. The study was published in Science. Cell [Internet].

Agency IQ
SEPTEMBER 22, 2023
Following a 2018 draft framework, FDA unveils long-awaited PDURS draft guidance A long-awaited draft guidance on Prescription Drug Use-Related Software (PDURS) outlines when information from digital health tools could be represented on prescription drug labeling. See AgencyIQ’s full analysis of the 2018 framework, and industry response, here.]

Agency IQ
JULY 22, 2024
The 103 regulations the EPA is currently working on On July 5, 2024, the EPA published its Spring 2024 Unified Agenda, which reveals what regulations the agency is planning on releasing by the end of the year and beyond.

Agency IQ
JULY 8, 2024
Supreme Court significantly expands time to file lawsuits over agency regulations The Supreme Court has issued its opinion in Corner Post v. In the Dodd-Frank Act, the Federal Reserve was tasked with exploring a cap on interchange fees, and it published Regulation II in 2011 , which set a maximum fee of $.021 021 per transaction plus.05%

Agency IQ
SEPTEMBER 15, 2023
PFAS regulation in California (late 2023 edition) California consistently maintains its status as both one of the most important economies in the world as well as one of the most regulated states in the United States. In 2019, the state began to significantly ramp up its PFAS regulation and research.

Broad Institute
JANUARY 22, 2024
Since 2018, the Cancer Dependency Map (DepMap) Consortium , an academic-industrial partnership launched by the Broad, has uncovered several potential drug targets by systematically screening cancer models in search of genetic dependencies. But we discovered two negative regulators [inhibitors of gene expression], DUSP4 and DUSP6.
Drug Target Review
JULY 7, 2023
The epigenome (meaning ‘above the genome’) is a system of reversible marks regulating how the DNA is read, translated and used. 2 Additionally, the epigenetic editing toolkit allows for precise temporal regulation. 2018;:15–25. What is epigenetic editing? We are on the precipice of a new era in genetic medicine.

Agency IQ
AUGUST 25, 2023
Cannabis groups to Congress: FDA should regulate CBD as a dietary supplement U.S. legislators have asked for help to reimagine how the FDA should regulate cannabidiol (CBD) following the agency’s determination that it could not make use of its existing legislative or regulatory authorities to do so.

Advarra
NOVEMBER 10, 2022
These notices proposed harmonization with the Health and Human Services (HHS) 2018 Common Rule (also referred to as the revised Common Rule ).?This In addition to the harmonization goal, these additional elements help modernize the regulations with current scientific and participant expectations. . Mandated Single IRB (sIRB) Review

FDA Law Blog: Biosimilars
JANUARY 15, 2024
Let’s just say, the smackdown—er, decision—eviscerates FDA’s approach to regulating flavored e-cigarettes. Says the Court: “It is perhaps possible that FDA did its part to give the regulated community clear guidance and that one million out of one million not only got it wrong but got it unreasonably wrong. FDA failed on each count.

Drug Target Review
SEPTEMBER 26, 2023
Ther Innov Regul Sci. 2018 Mar;17(3):167–81. 2018 Dec 11;94(10):390–411. 2018;(136):56573. Regul Toxicol Pharmacol RTP. Strauss DG, Gintant G, Li Z, Wu W, Blinova K, Vicente J, et al. 2018;53(4):519–25. Colatsky T, Fermini B, Gintant G, Pierson JB, Sager P, Sekino Y, et al. Nat Rev Drug Discov. 2016;81:37–46.

FDA Law Blog: Biosimilars
SEPTEMBER 11, 2023
Baumhardt, Senior Medical Device Regulation Expert & Adrienne R. Lenz, Principal Medical Device Regulation Expert — On September 6, 2023, FDA announced its latest efforts to modernize the 510(k) process, outlining FDA’s latest improvements to strengthen the 510(k) Program and announcing release of three draft guidance documents.

Advarra
DECEMBER 1, 2022
In the past several years, Health and Human Services (HHS) Office of Human Research Protections (OHRP), the Food and Drug Administration (FDA), and the National Institutes of Health (NIH) have announced and/or implemented new regulations to address the challenges of conducting clinical trials involving multiple research sites. An Age of sIRB.

FDA Law Blog: Biosimilars
MAY 13, 2024
Gibbs & Ana Loloei & Véronique Li, Senior Medical Device Regulation Expert — FDA has long touted the use of real-world evidence ( RWE ). In fact, in 2018, FDA emphasized that leveraging the use of RWD and RWE in regulatory decision-making is “a top strategic priority for the FDA.” By Jeffrey N.

Drug Target Review
FEBRUARY 28, 2024
Trends Immunol 39(6):446-459 (2018). Nat Commun 9(1):1760 (2018). Regulation of cutaneous malignancy by gammadelta T cells. J Front Immunol 9:1409 (2018). Davey M, Willcox C, Baker A, et al. Recasting human Vδ1 lymphocytes in an adaptive role. Davey M, Willcox C, Hunter S, et al. Kabelitz D, Serrano R, Kouakanou L, et al.

Drug Channels
DECEMBER 17, 2020
HHS relied in part on a highly misleading ASPE study of 2018 data. Last year, Dr. Scott Gottlieb, a former FDA commissioner, argued that we shouldn’t give up on biosimilars and prematurely regulate prices. Drug pricing perceptions always seem to lag reality. Consider HHS's "Most Favored Nation" model for Medicare Part B.

Perficient: Drug Development
MARCH 26, 2024
Public Sector Regulations : Public sector bodies are mandated to comply with accessibility regulations like the Public Sector Bodies (Websites and Mobile Applications) Accessibility Regulations 2018 in the UK. Meeting the WCAG 2.2 AA standard is essential for compliance.
Drug Target Review
JULY 13, 2023
Dewpoint Therapeutics, a platform drug discovery company founded in 2018, has been at the forefront of this exciting field. As for regulatory considerations, Klein anticipates engaging in discussions with regulators to address novel aspects such as mechanism, toxicology, and dosing regimens.

FDA Law Blog: Biosimilars
DECEMBER 11, 2023
1] Yet FDA’s conclusions about the Agency’s ability to regulate the entire laboratory industry are based on fundamentally flawed assumptions about the number of entities and tests that will be subject to FDA regulation. This is based on FDA’s reported 2018 review of the CLIA database. [2]

The Pharma Data
APRIL 6, 2021
persons outside the United States in reliance on Regulation S under the Securities Act. In member states of the European Economic Area, this announcement is directed only at persons who are “qualified investors” within the meaning of the Prospectus Regulation.

FDA Law Blog: Biosimilars
JULY 25, 2023
Recent industry comments submitted to FDA and new, international efforts against these nefarious, potentially carcinogenic organic compounds have the shifting state of regulation here back in the news. The evolving upswing in NDSRI regulation is due to their link to cancer. Koblitz — We need to talk about nitrosamines.

FDA Law Blog: Biosimilars
AUGUST 12, 2024
As with other FDA-regulated products, such as human drugs and medical devices, the “regulatory review period” is composed of a “testing phase” and a “review phase.” FDA’s PTE regulations at 21 C.F.R. FDA explicitly said as much in the 1991 preamble accompanying proposed patent restoration regulations.
The Pharma Data
APRIL 8, 2021
persons outside the United States in reliance on Regulation S under the Securities Act. In member states of the European Economic Area, this announcement is directed only at persons who are “qualified investors” within the meaning of the Prospectus Regulation.

DrugBank
OCTOBER 4, 2024
However, ensuring that these structures comply with applicable tax laws and regulations is essential to avoid potential legal and reputational risks. For example, Takeda Pharmaceutical's $62 billion acquisition of Shire plc in 2018 expanded its global footprint and tapped into Shire's strong presence in emerging markets.

Drug Target Review
OCTOBER 31, 2023
Regulatory affairs workers get to tell a story about a drug to the regulators and – keeping the end in mind, which is the drug label or prescribing information – is a good way to communicate with regulators. Reneo has been working on this since before 2018. Every drug has a story: an end game.

Broad Institute
AUGUST 31, 2023
Through the imposition of a user-defined GRN in its architecture, GRouNdGAN simulates steady-state and transient-state single-cell datasets where genes are causally expressed under the control of their regulating transcription factors (TFs).

Advarra
DECEMBER 15, 2022
Policies and Regulations Affecting Single IRB Review. with compliance required beginning January 25, 2018. Health and Human Services (HHS) regulations as much as practicable, which includes sIRB requirements. Working with an sIRB remains completely voluntary in many cases. The policy doesn’t apply to foreign sites.

Agency IQ
FEBRUARY 15, 2024
The regulation of BPA in the EU today BPA has been jointly registered (as a full registration) under REACH with over 60 registrants manufacturing or importing the substance in the tonnage band at or above 1,000,000 metric tons per year. B1) BPA’s regulation across so many sectors affecting different parts of the supply chain (i.e.,

Agency IQ
MAY 10, 2024
The new guidance maintains the agency’s existing definitions of remanufacturing and servicing, but adds a new section that provide a high level overview of the medical device regulations – primarily for those “less familiar.” Also this year, the FDA finalized its new rule on overhauling the medical device quality system regulations.

NIH Director's Blog: Drug Discovery
MAY 9, 2019
Among those taking on this ambitious challenge is a recipient of a 2018 NIH Director’s New Innovator Award, Srivatsan Raman of the University of Wisconsin-Madison.
NIH Director's Blog: Drug Development
SEPTEMBER 20, 2018
That includes those involved in regulating our appetites and our moods, via a class of G proteins known as inhibitory G proteins (G i ). 2018 Jun;558(7711):553-558. [2] 2018 Apr;93(4):251-258. [3] They share a common architecture and bind a relatively limited number of intracellular signaling proteins called G proteins.

Broad Institute
JUNE 14, 2023
A trailblazer in cancer research, Glimcher's research identified key transcriptional regulators of protective immunity and the origin of pathophysiologic immune responses underlying autoimmune, infectious, and malignant diseases. She is also a member of the board of trustees of the Massachusetts Health & Hospital Association.

Advarra
DECEMBER 14, 2023
Advarra analysis of the FDA Inspection Observation Datasets from 2018 – 2022 shows the following causes make up 44% of all findings: Failure to Follow Investigational Plan (21 CFR 312.60) Citations for failure to follow the investigational plan contribute to over half of the failures recorded since 2018.
Drug Target Review
AUGUST 24, 2023
She joined the company in November 2018 with more than 10 years of experience in drug discovery and non-clinical development of immunomodulatory drugs in the immuno-oncology space. Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Cancer Immunology Research. 2021 Dec 1;9(12):1425-38.

The Pharma Data
SEPTEMBER 3, 2020
The world’s first gene-edited babies were born in China in November 2018. Most countries have regulations in place preventing babies being born after gene-editing, but the incident led to calls for strong international consensus. The scientist responsible was jailed, amid a fierce global backlash.
Drug Hunter
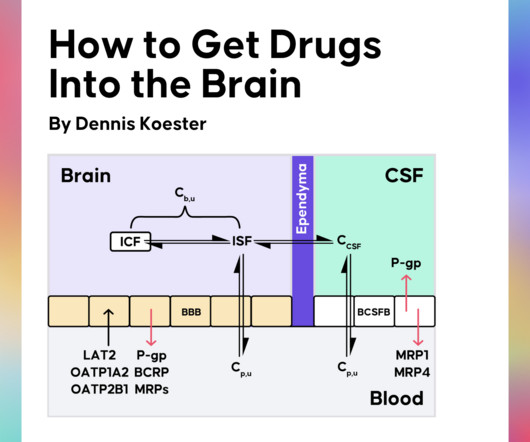
AUGUST 21, 2023
The BBB is composed of specialized brain endothelial cells that regulate the distribution of molecules into and out of the brain from the blood. Unlike the endothelial BBB, the blood-cerebrospinal fluid-barrier (BCSFB) is made up of epithelial cells that similarly regulate the distribution of molecules between the blood and CSF.

Drug Target Review
MAY 23, 2023
The algae-derived non-digestible oligosaccharides can also influence the expression of cytokines that regulate the immune system. Regulation (EU) 2017/2470 maintains this list within the novel food catalogue. The legislation governing this list is for consumer protection and also applies to algae that are intended for use as food.

The Pharma Data
MARCH 1, 2022
Syndesi’s unique molecules act pre-synaptically to enhance synaptic efficiency by positively modulating the function of synaptic vesicle protein 2A (SV2A), which plays a central role in regulating neurotransmission. The lead molecule, SDI-118, was discovered by UCB before being out-licensed to Syndesi as of 2018.

The Pharma Data
JULY 28, 2021
She will serve as Chair of the Board of Directors upon transformation of EUROAPI into a société anonyme , in compliance with applicable corporate governance regulations. She also served on the Board of Idorsia from 2018 to 2021.

Agency IQ
AUGUST 23, 2024
The office, which is responsible for directly reviewing and regulating medical devices and diagnostics, was established under a major restructuring of CDRH in 2018-2019 as part of CDRH’s “Total Product Lifecycle (TPLC)” approach. Stenzel had joined the agency as Office Director in 2018 and helmed the OHT7 through the pandemic.

The Pharma Data
MARCH 3, 2022
In 2018, Shalev and colleagues reported the benefits of verapamil in a one-year clinical study of Type 1 diabetes patients, finding that regular oral administration of verapamil enabled patients to produce higher levels of their own insulin, thus limiting their need for injected insulin to regulate blood sugar levels.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content