GSK antibody drug reduces COPD attacks in trial
BioPharma Drive: Drug Pricing
SEPTEMBER 6, 2024
New trial results could offer support for an expansion of Nucala’s label after U.S. regulators rejected GSK’s submission in 2018.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
SEPTEMBER 6, 2024
New trial results could offer support for an expansion of Nucala’s label after U.S. regulators rejected GSK’s submission in 2018.

Drugs.com
OCTOBER 1, 2024
1, 2024 -- Clinical trials sponsored by Big Pharma enrolled eight times as many patients as U.S.-government government trials did between 2018 and 2022, new research shows.The study -- conducted by researchers at Fred Hutch Cancer Center in. TUESDAY, Oct.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

BioPharma Drive: Drug Pricing
OCTOBER 22, 2024
A medicine the pharma acquired in a $430 million buyout of Visterra in 2018 succeeded in a Phase 3 trial in IgA nephropathy, a crowded corner of drug research.

Antidote
JULY 29, 2022
Between 2009 and 2018, U.S. The process of getting a new drug to market is an expensive one. biopharmaceutical companies spent about $1 billion per drug according to an analysis published in JAMA , and other studies have found that it can cost up to $2.8 billion to bring a new therapy to market.

Codon
NOVEMBER 3, 2024
Still, while these developments provide cause for hope, each drug or vaccine faces a variety of challenges, ranging from financial incentives for clinical trials to the difficulty of discovering new antimicrobials that pass safety and efficacy tests. A phase 3 clinical trial for M72/AS01E, funded by the Gates Foundation, began this year.

Antidote
NOVEMBER 20, 2023
In both 2018 and 2023, Antidote partnered with SCORR marketing to conduct surveys intended to increase the understanding of patients’ perceptions of clinical trials. Our 2018 survey collected data from nearly 4,000 individuals to gain insight into what matters most when considering a clinical trial.

Drug Target Review
NOVEMBER 1, 2023
2 Implication of ATX in a large range of human diseases have been highlighted by both fundamental research and clinical trials. 1-5 Implication of ATX in a large range of human diseases have been highlighted by both fundamental research and clinical trials. 2018 Jun 13;5. References: Ninou I, Magkrioti C, Aidinis V. J Med Chem.

Antidote
SEPTEMBER 15, 2023
In the third quarter of 2018, we conducted a survey with SCORR Marketing to learn more about the patient perception of clinical trial participation.

Antidote
NOVEMBER 10, 2023
In 2018, we partnered with SCORR Marketing to conduct a survey aimed at gaining knowledge about the patient perception of clinical trial participation. We collected nearly 4,000 responses from individuals regarding what matters most to when considering a clinical trial.

FDA Law Blog: Drug Discovery
JULY 17, 2024
An Opportunity to Leverage the Newly Created Rare Disease Advisory Committee across CDER and CBER In Frank and James’ 2018 proposal there was also a recommendation for the formation of a Rare Disease Advisory Committee, which would allow FDA access to experts in the science of small trials and other aspects of rare disease research.

Vial
MAY 13, 2024
However, progress from molecule to approved drug is hampered by extremely high costs and lengthy clinical trials , and approximately 90% of drugs that reach clinical trials fail. only 5% of molecules in oncology Phase I trials reach the market taking, on average, 7.5 An estimated 80% of clinical trials do not finish on time.

The Pharma Data
JUNE 26, 2021
Sanofi and Translate Bio initiate Phase 1 clinical trial of mRNA influenza vaccine. The trial will evaluate the safety and immunogenicity of a monovalent flu vaccine candidate coding for the hemagglutinin protein of the A/H3N2 strain of the influenza virus. JUNE 22 , 2021.

Advarra
OCTOBER 19, 2023
The clinical trial startup process has seen significant shifts over the past five years: growth in decentralized trials, improved technology, and an increased demand for accelerated timelines in getting therapies to market. Growth in Volume About 60% of survey respondents said their study volume is higher now than compared to 2018.

Conversations in Drug Development Trends
FEBRUARY 28, 2023
Over the next few decades, that drug made its way through clinical trials, securing approval in 2007—just 36 months before Rob was diagnosed in 2010. In 2018, we partnered with Project ALS to fund research at Columbia University into synthetic motor neurons in ALS patients. But what was truly remarkable?

The Pharma Data
NOVEMBER 2, 2020
The SHINE trial is a phase 3 randomised open-label trial comparing four versus six months of treatment with rifampicin, isoniazid, pyrazinamide plus or minus ethambutol in children with smear-negative, non-severe TB. 1204 children aged under 16 years participated in the trial, including 127 children living with HIV infection.

The Pharma Data
MARCH 11, 2021
Clinical trial to assess safety, immune response and reactogenicity, after preclinical data showed high neutralizing antibody levels. The Companies expect interim results from this trial in the third quarter of 2021. About the Phase 1/2 clinical trial. Expected to enroll 415 participants; interim results expected in Q3 2021.

FDA Law Blog: Biosimilars
MAY 13, 2024
FDA has even gone so far as to maintain that the “real-life clinical performance of a medical product might be more clearly demonstrated through RWD/RWE because a controlled clinical trial often cannot evaluate all applications of a product in clinical practice across the full range of potential users.”
The Pharma Data
SEPTEMBER 2, 2021
The Phase 3 RENOIR trial of RSVpreF is a global, randomized, double-blind, placebo-controlled study that expects to enroll approximately 30,000 participants 60 years and older. NEW YORK–(BUSINESS WIRE)– Pfizer Inc. Detailed results from the study will be shared at a future medical conference. Source link: [link].

Agency IQ
SEPTEMBER 22, 2023
Following a 2018 draft framework, FDA unveils long-awaited PDURS draft guidance A long-awaited draft guidance on Prescription Drug Use-Related Software (PDURS) outlines when information from digital health tools could be represented on prescription drug labeling. See AgencyIQ’s full analysis of the 2018 framework, and industry response, here.]

Codon
JANUARY 21, 2024
Human challenge trials were an indispensable part of the development of the malaria vaccine, R21/Matrix-M, endorsed by the World Health Organization last October. But for all of their benefits, human challenge trials have their drawbacks. Jake himself has participated in both Zika and Shigella challenge trials.
PLOS: DNA Science
MARCH 21, 2024
A Long and Winding Diagnostic Road The story begins in 2011, with Alessandra Biffi, MD, head of the clinical trial for MLD at the San Raffaele Telethon Institute for Gene Therapy in Milan. I told the story of one family with MLD, the Prices, as part of a post celebrating the moms of gene therapy in 2018. She passed away in 2013.

The Pharma Data
AUGUST 22, 2021
The trial accrued 25 cases of symptomatic COVID-19 at the primary analysis.There were no cases of severe COVID-19 or COVID-19-related deaths in those treated with AZD7442. The trial included 5,197 participants in a 2:1 randomisation AZD7442 to placebo. The trial was conducted in 87 sites in the US, UK, Spain, France and Belgium.
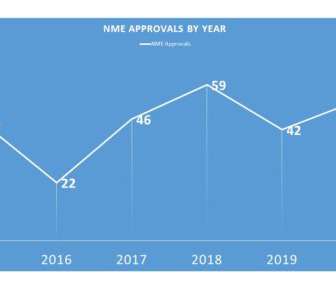
Eye on FDA
JANUARY 6, 2021
During 2020, FDA was able to approve 53 – not surpassing the 2018 all time record of 59, but certainly an admirable second place. One might have surmised that the advent of the COVID-era might have thrown off new approvals and that NMEs approved would go diminish. That, however, was not really the case. Happy New Year, by the way.

The Pharma Data
JANUARY 13, 2021
PITTSBURGH–( BUSINESS WIRE )– Knopp Biosciences LLC today announced positive top-line results in a Phase 2 dose-ranging trial of the novel oral drug dexpramipexole in patients with moderate-to-severe eosinophilic asthma. The trial was conducted at 28 U.S. 14, 2021 11:00 UTC. study centers. ABOUT THE EXHALE STUDY.

PPD
SEPTEMBER 6, 2023
Approaches to outsourcing clinical trials have changed significantly in recent years. In 2018, market utilization of FSO models was at 72%, with usage of FSP models lagging at 28%. Growth of the FSP market is steadily increasing. But in just three years, FSP usage has reached 41%. Overseen by an insourced project manager.

The Pharma Data
MARCH 16, 2022
The Independent Data Monitoring Committee recommended that the EMPA-KIDNEY trial be stopped early, following a formal interim assessment EMPA-KIDNEY is the largest and broadest dedicated SGLT2 inhibitor trial in chronic kidney disease to date Detailed results are expected to be presented later this year.
Drug Target Review
JANUARY 25, 2024
The peptide in development is based on a 2018 Cleveland Clinic discovery and serves as a proof-of-concept for this type of drug for triple-negative breast cancer. Currently, they are exploring the drug’s potential for clinical trials. 1 Some peptides supplement existing proteins in the body, like insulin for people with diabetes.
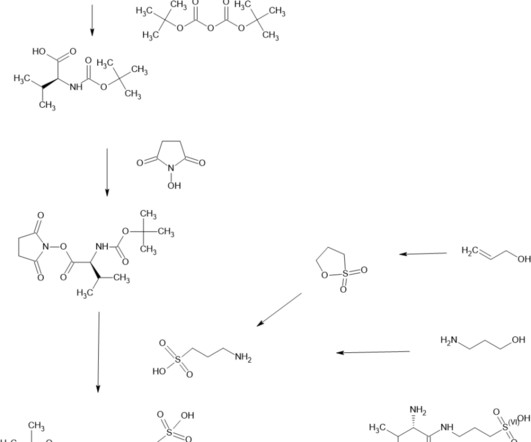
New Drug Approvals
OCTOBER 12, 2024
Years later, a subgroup analysis of the trial data indicated a potential positive effect in participants who carried two copies of ApoE4 ( Abushakra et al., 2018; 32(9): 849–861. [2]. 2018 Mar;57(3):315-333. After tramiprosate failed in Phase 3, its maker, NeuroChem, marketed it as a nutritional supplement. Hey JA, et al.
The Pharma Data
MARCH 15, 2021
Trial enrolled patients with advanced cervical cancer regardless of PD-L1 status. The trial will be stopped early based on a unanimous recommendation by the Independent Data Monitoring Committee (IDMC), and the data will form the basis of regulatory submissions in 2021. This is reflected in the trial where the average age was 51.”.

The Pharma Data
NOVEMBER 9, 2020
Trial Also Met the Primary Endpoint in Patients With Low Levels of Eosinophils. In the subgroup of patients with baseline eosinophil counts less than 300 cells per microliter, the trial met the primary endpoint with tezepelumab demonstrating a statistically significant and clinically meaningful reduction in AAER.

The Pharma Data
JANUARY 31, 2021
Adjuvanted S-Trimer COVID-19 vaccine candidates demonstrated favorable safety and tolerability profiles and strong neutralizing immune responses in a phase 1 trial. Clover plans to initiate a global phase 2/3 trial in the first half of 2021 with an interim analysis for vaccine efficacy potentially in the middle of 2021.

The Pharma Data
JANUARY 27, 2021
a clinical stage biotech company focused on developing novel treatments for neurodegenerative and neurodevelopmental disorders, today announces the enrollment of the first participant in Europe in its global Phase 3 clinical trial for HD. The first US subjects in the PROOF-HD trial were enrolled in October 2020.

Eye on FDA
AUGUST 4, 2021
When Breakthrough Therapy (BT) designation is granted, then the drug gets Fast Track status and receives more intensive guidance – which can begin as early as Phase I clinical trials. Fewer meetings – more approvals.

Drug Target Review
OCTOBER 11, 2023
Symptoms were reversed in mouse models and a clinical trial is planned for later this year. 5 This drug candidate is progressing towards the clinic, with phase 1 trials due to start within 18 months. 2018 [cited 2023 Sep 5]. This restores movement and improves nerve condition, which should bring back sensation.

Codon
JULY 14, 2024
However, the subsequent death of another patient thrust Denys into a contentious trial. Despite the high costs, researchers in Kyoto generated enough cultured platelets in 2022 to transfuse a 55-year-old woman for an initial human trial. The results of the trial are expected at the end of 2024. Data from Rousseau G.F.

New Drug Approvals
SEPTEMBER 29, 2024
2018 ; Ding et al., Findings Two Phase 1 trials of atuzaginstat were completed by June 2019. According to a company press release and a poster presentation at the 2018 CTAD conference, healthy adults received 25, 50, or 100 mg COR388 or placebo every 12 hours for 10 days; AD patients took 50 mg or placebo every 12 hours for 28 days.

The Pharma Data
DECEMBER 27, 2020
In these unprecedented times, we are especially grateful for the dedication of our research site staff and trial participants, whose commitment to EXCELLENCE facilitated the advancement of our lead program,” said George Yeh, President of TLC. “We SOUTH SAN FRANCISCO, Calif. and TAIPEI, Taiwan, Dec.

The Pharma Data
JANUARY 14, 2021
(NASDAQ: FPRX) today announced clinical results from the global, randomized, double-blind placebo-controlled Phase 2 FIGHT trial evaluating first-in-class targeted therapy bemarituzumab in advanced gastric or gastroesophageal junction (GEJ) cancer. Phase 2 FIGHT Trial: Summary of Efficacy*. Wainberg, M.D., .

The Pharma Data
DECEMBER 21, 2020
21, 2020 /PRNewswire/ — Amgen (NASDAQ:AMGN) and AstraZeneca today announced the SOURCE trial did not meet the primary endpoint of a statistically significant reduction in the daily oral corticosteroid (OCS) dose, without loss of asthma control, with tezepelumab compared to placebo. THOUSAND OAKS, Calif. ,

Drug Target Review
FEBRUARY 28, 2024
Preclinical research on γδ T cells has made great strides since the cells were first identified in the 1980s, with γδ T-cell therapies from several companies, including IN8bio, now in or nearing clinical trials for various cancers. Trends Immunol 39(6):446-459 (2018). Nat Commun 9(1):1760 (2018). J Front Immunol 9:1409 (2018).

The Pharma Data
SEPTEMBER 10, 2020
Pascal Soriot, Chief Executive of AstraZeneca, said that the company’s vaccine could still be available this year, despite the clinical trials being paused after a participant became ill. . Soriot has stressed that it is common practice to halt clinical trials for such reasons, but was unable to say when the trial would get back underway.

FDA Law Blog: Biosimilars
JANUARY 15, 2024
FDA said randomized clinical trials could be used, but so could observational studies with respect to cessation data. While FDA did try to suggest that these studies were not a requirement, the Court cited a PMTA checklist in FDA’s Decisions Document that included a requirement for a randomized controlled trial or a longitudinal cohort study.

The Pharma Data
DECEMBER 31, 2020
Tabibiazar co-founded private Aravive Biologics and served as the Chairman of its board of directors and as President and Chief Executive Officer from its inception to April 2017 and as Executive Chairman from May 2017 until October 2018. since October 2018. to form the combined company, Aravive, Inc.

Advarra
DECEMBER 14, 2023
Sponsor study team members and research sites each play a critical role in clinical trial executions. Both inspection findings and inspection failures could reduce the quality of the trial data, with inspection failures being most disruptive. In addition, many research trials are conducted over the course of many years.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content