Concert Pharmaceuticals Reports Third Quarter 2020 Financial Results and Provides Company Update
The Pharma Data
NOVEMBER 4, 2020
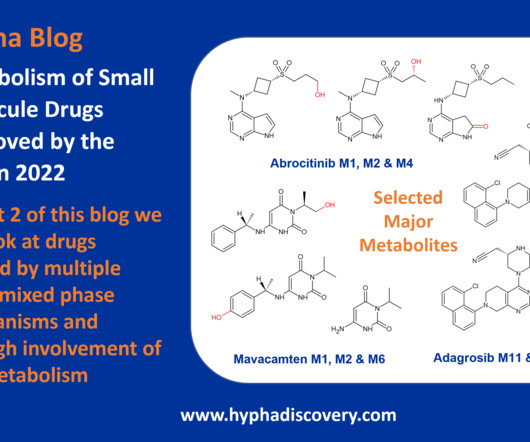
million as of December 31, 2019. Research and development expenses were $16.3 million for the same period in 2019. The increase in research and development expenses relates primarily to the ongoing Phase 2 clinical trial of CTP-692. million for the same period in 2019. 2019.













Let's personalize your content