Kala Gets the Greenlight from FDA for Dry Eye Disease Treatment
The Pharma Data
OCTOBER 26, 2020
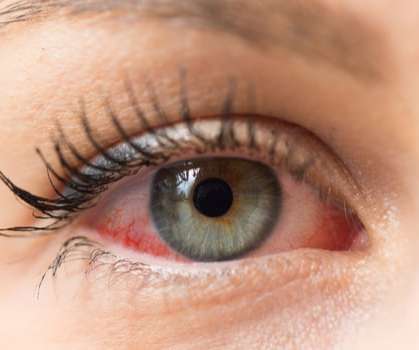
Food and Drug Administration (FDA) has approved EYSUVIS for the short-term treatment of dry eye disease. . adults have been diagnosed with dry eye disease, a chronic, episodic, multifactorial disease. EYSUVIS is the first FDA-approved corticosteroid specifically for dry eye disease treatment.
































Let's personalize your content