Kala Gets the Greenlight from FDA for Dry Eye Disease Treatment
The Pharma Data
OCTOBER 26, 2020
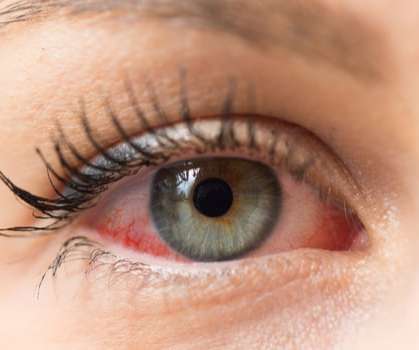
EYSUVIS is the first FDA-approved corticosteroid specifically for dry eye disease treatment. The corticosteroid then works to relieve swelling, redness and itching by targeting the immune response that drives dry eye disease flares. . Over-the-counter treatments fail due to the body’s natural protective system.




























Let's personalize your content