‘Ice Bucket Challenge’ funds FDA-approved ALS treatment
Drug Discovery World
OCTOBER 4, 2022
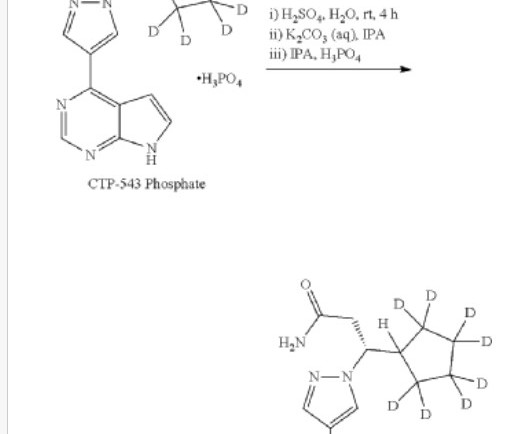
The US Food and Drug Administration (FDA) has granted approval to AMX0035, a new treatment for people living with amyotrophic lateral sclerosis (ALS). million from the ALS Association, raised by the 2014 social media craze the ‘Ice Bucket Challenge’ Another 40 potential new treatments are being investigated. .












































Let's personalize your content