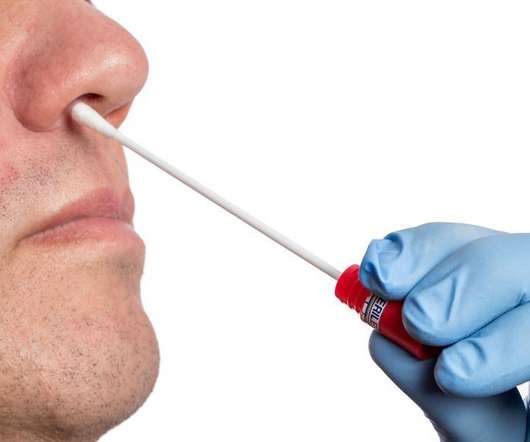
FDA Approves First Over-the-Counter Fully At-Home Test for COVID-19
The Pharma Data
DECEMBER 15, 2020
FDA Approves First Over-the-Counter Fully At-Home Test for COVID-19. 15, 2020 — The first nonprescription COVID-19 test that enables people to collect samples and get results at home has received emergency use authorization from the U.S. Ellume expects to produce more than 3 million tests in January 2021, the FDA said.















































Let's personalize your content