SRP-001: redefining pain treatment with a safer, non-opioid analgesic
Drug Target Review
JULY 1, 2024
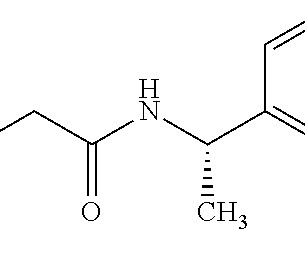
As a non-opioid, SRP-001 also eliminates abuse potential, positioning it as a safer and effective drug candidate for the treatment of acute and neuropathic pain and migraine headache. How does SRP-001’s safety profile, especially concerning hepatotoxicity and nephrotoxicity, compare to other common pain medications?












































Let's personalize your content