Metabolism of 2023 FDA Approved Small Molecules – PART 2
Metabolite Tales Blog
APRIL 10, 2024
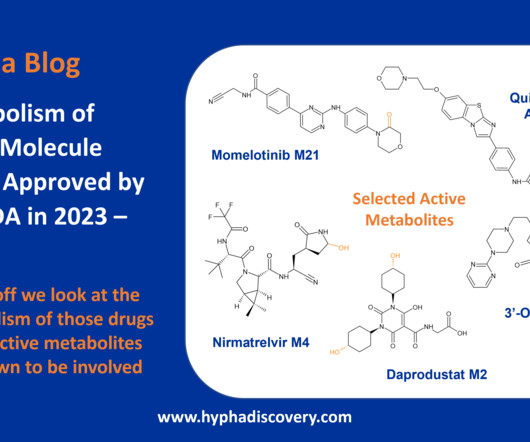
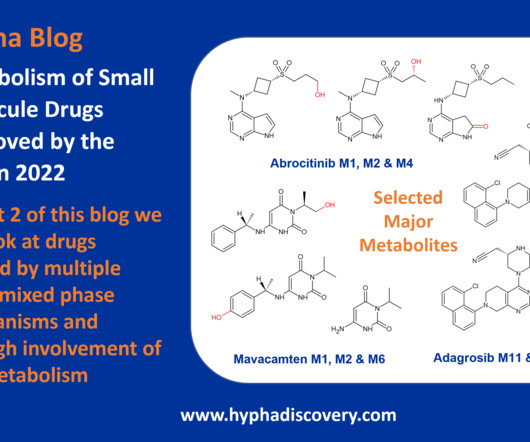
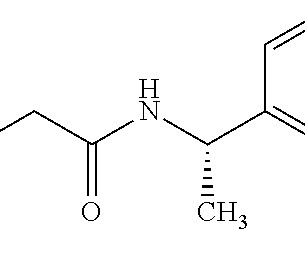
Table 1: Small molecule drugs approved by the FDA in 2023 with reported involvement of phase II mechanisms In vitro : In vivo differences Incubation of the SGLT2 (sodium-glucose co-transporter-2) inhibitor bexagliflozin in human liver microsomes points to metabolism through both oxidation and glucuronidation to 6 main metabolites.














































Let's personalize your content