Approvals in the Crazy Year of 2020
Eye on FDA
JANUARY 6, 2021
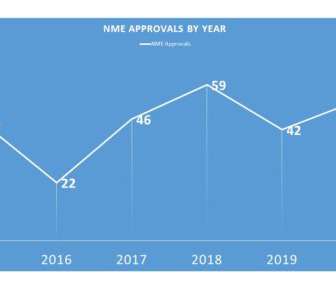
During 2020, FDA was able to approve 53 – not surpassing the 2018 all time record of 59, but certainly an admirable second place. Notably, the 2020 NME approvals included 22 related to oncology, with 2 new GIST treatments, 3 in breast cancer and 2 approvals in prostate cancer. That, however, was not really the case.







































Let's personalize your content