Simple test for flu could improve diagnosis and surveillance
Broad Institute
JUNE 18, 2024
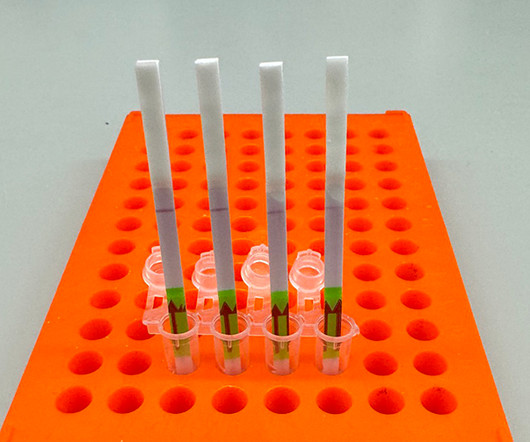
Simple test for flu could improve diagnosis and surveillance By Allessandra DiCorato June 18, 2024 Breadcrumb Home Simple test for flu could improve diagnosis and surveillance A low-cost CRISPR-based paper strip test distinguishes between influenza types and can be reprogrammed to recognize different viruses including the H5N1 bird flu virus.









































Let's personalize your content