Biogen, Sage tremor drug fails key trial
BioPharma Drive: Drug Pricing
JULY 24, 2024
Known as SAGE-324, the drug was one of the key assets Biogen gained rights to through a billion-dollar research deal inked back in 2020.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
JULY 24, 2024
Known as SAGE-324, the drug was one of the key assets Biogen gained rights to through a billion-dollar research deal inked back in 2020.

PPD
AUGUST 16, 2023
The COVID-19 pandemic rapidly accelerated the adoption of hybrid and decentralized clinical trial (DCT) models. However, as the world settles into its post-pandemic state and returns to pre-pandemic paradigms in many areas, the pharmaceutical industry remains dedicated to moving beyond traditional, centralized clinical trial constructs.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Patent Watch
DECEMBER 30, 2024
Types of Drug Applications The PMDA accepts three main types of drug applications: Investigational New Drug (IND) : Required for conducting clinical trials in Japan. Challenges and Opportunities Ethnic Bridging : Japan has historically required clinical trials to be conducted within the country to account for ethnic differences.
Eye on FDA
JANUARY 6, 2021
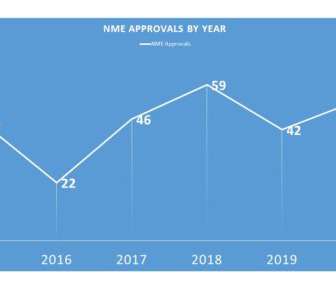
During 2020, FDA was able to approve 53 – not surpassing the 2018 all time record of 59, but certainly an admirable second place. Notably, the 2020 NME approvals included 22 related to oncology, with 2 new GIST treatments, 3 in breast cancer and 2 approvals in prostate cancer. That, however, was not really the case.

Drug Discovery Today
NOVEMBER 10, 2020
Solna, Sweden, November 10, 2020. Affibody AB (“Affibody”), a clinical stage biopharmaceutical company, today announced the initiation of its 52-week trial investigating the novel bispecific IL-17A inhibitor ABY-035 in patients with psoriatic arthritis (PsA).

Codon
NOVEMBER 3, 2024
Still, while these developments provide cause for hope, each drug or vaccine faces a variety of challenges, ranging from financial incentives for clinical trials to the difficulty of discovering new antimicrobials that pass safety and efficacy tests. A phase 3 clinical trial for M72/AS01E, funded by the Gates Foundation, began this year.

National Institute on Drug Abuse: Nora's Blog
MARCH 6, 2025
Advancing reduction of drug use as an endpoint in addiction treatment trials astewart Thu, 03/06/2025 - 09:59 Nora's Blog March 18, 2025 Image Getty Images/ SolStock This blog was also published in the American Society of Addiction Medicine (ASAM) Weekly on March 18, 2025.&

FDA Law Blog: Drug Discovery
MAY 4, 2023
Cato — On May 2nd, FDA released a new draft guidance with recommendations for decentralized clinical trials (DCTs) for drugs, biologics, and devices. In a DCT, trial-related activities may occur in trial participants’ homes, at local health care providers’ offices, or in local clinical laboratories.

ProRelix Research
SEPTEMBER 21, 2022
According to the World Health Organization (WHO), cancer is the leading cause of death worldwide, with a death rate of one in six in 2020 (1). The post Cancer Clinical Trials: USA Scenario and Study Designs appeared first on ProRelix Research. Aside from the high […].

Drug Target Review
FEBRUARY 10, 2025
In just two years, CTMC has advanced eight therapies into clinical trials, harnessing genetic engineering to enhance T-cell effectiveness in the fight against cancer. Around 2020, Bock assumed the challenge of creating a new division at MD Anderson focused on advancing cell therapies by bridging the gap between research labs and the clinic.

PPD
DECEMBER 16, 2024
Cost and complexity go hand-in-hand The rising costs and growing complexity in clinical trials are deeply linked, with patient recruitment, extended timelines and meeting regulatory demands emerging as some of the key drivers. Nearly half (49%) of clinical trial sponsors surveyed identified rising costs as their foremost concern in 2024.

FDA Law Blog: Drug Discovery
OCTOBER 11, 2023
Snow — On September 18, 2023, FDA published an updated, final iteration of guidance for immediate implementation entitled, “ Considerations for the Conduct of Clinical Trials of Medical Products During Major Disruptions Due to Disasters and Public Health Emergencies.” hurricane) or public health emergency (e.g.,

Drug Target Review
NOVEMBER 1, 2023
2 Implication of ATX in a large range of human diseases have been highlighted by both fundamental research and clinical trials. 1-5 Implication of ATX in a large range of human diseases have been highlighted by both fundamental research and clinical trials. He is a researcher at University of Oldenburg, Germany. Frontiers in Medicine.

Drug Target Review
JULY 4, 2023
The drivers behind the demand for ADCs Providing more targeted therapeutic options in the oncology space has underpinned the rapid growth of the ADC market; between 2016 to 2020, the US ADC market grew by 45 percent to a value of nearly $1.5 3D rendering of Antibody Drug Conjugate Molecules.

The Pharma Data
JUNE 26, 2021
Sanofi and Translate Bio initiate Phase 1 clinical trial of mRNA influenza vaccine. The trial will evaluate the safety and immunogenicity of a monovalent flu vaccine candidate coding for the hemagglutinin protein of the A/H3N2 strain of the influenza virus. JUNE 22 , 2021.

Vial
FEBRUARY 29, 2024
Contract research organizations (CROs) are an integral partner of the drug development process, as they play a pivotal role supporting clinical trial conduct for pharmaceutical, biotechnology, and medical device sponsor companies. That is, how many clinical trials are actually managed by these organizations?
Drug Target Review
OCTOBER 30, 2024
Now take a step further: envision testing drugs in these organoids to identify the ones that can treat disease safely and effectively without needing to run expensive clinical trials first. MedComm (2020). Further still, think about implanting these mini organs into the patient to restore lost function. 2009;459(7244):262-5.

The Pharma Data
AUGUST 22, 2021
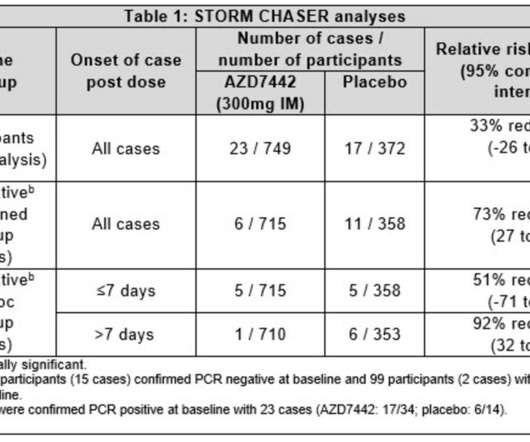
The trial accrued 25 cases of symptomatic COVID-19 at the primary analysis.There were no cases of severe COVID-19 or COVID-19-related deaths in those treated with AZD7442. The trial included 5,197 participants in a 2:1 randomisation AZD7442 to placebo. The trial was conducted in 87 sites in the US, UK, Spain, France and Belgium.

Agency IQ
JULY 22, 2024
Halfway there: Novel drug approvals and their supportive clinical trials so far in 2024 In the first half of 2024, the FDA’s Center for Drug Evaluation and Research (CDER) approved 21 novel drug products. Most NMEs approved so far this year relied on one pivotal trial.

DrugBank
JULY 20, 2023
We are excited to bring FDA Orphan data into DrugBank because we know that at the earliest stages of drug discovery, curating reliable scientific data, identifying relevant previous trials, and designing protocols for success are all important, challenging, and time-consuming tasks. of trials stopped due to recruitment issues.
The Pharma Data
JUNE 25, 2021
AstraZeneca today announced results from the STORM CHASER trial assessing the safety and efficacy of AZD7442, a long-acting antibody (LAAB) combination, for the prevention of symptomatic COVID-19 in participants recently exposed to the SARS-CoV-2 virus. The PROVENT trial will give us more clarity in this patient population.

The Pharma Data
JULY 21, 2021
(NYSE: PFE), today announced that they have completed recruitment for the Phase 2 trial, VLA15-221, of Lyme disease vaccine candidate, VLA15. The trial builds on previous positive Phase 2 trials and includes both adult and pediatric participants with the aim to support acceleration of the vaccine candidate’s pediatric program.
The Pharma Data
JUNE 25, 2021
To identify strains causing COVID-19 infections within the trial, sequencing of virus variants has so far been performed on 474 COVID-19 cases, of which 124 fulfilled adjudication criteria and were included in the present efficacy analysis. CureVac began development of mRNA-based COVID-19 vaccine candidates in January 2020.
The Pharma Data
APRIL 3, 2021
The CoVIg-19 Plasma Alliance today announced that the Phase 3 Inpatient Treatment with Anti-Coronavirus Immunoglobulin (ITAC) clinical trial sponsored and funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), did not meet its endpoints. About the ITAC Trial.
The Pharma Data
SEPTEMBER 2, 2021
The Phase 3 RENOIR trial of RSVpreF is a global, randomized, double-blind, placebo-controlled study that expects to enroll approximately 30,000 participants 60 years and older. NEW YORK–(BUSINESS WIRE)– Pfizer Inc. The results of the study enabled Pfizer to proceed to Phase 3.
The Pharma Data
AUGUST 30, 2021
Results from the Phase III EMPEROR-Preserved trial were presented today at the European Society of Cardiology Congress 2021 2 and published in The New England Journal of Medicine 1. 1 Trial participants were randomly assigned to empagliflozin 10 mg (n=2,997) or placebo (n=2,991) once daily.

The Pharma Data
JULY 15, 2021
Trial will include participants at risk for cognitive and functional decline related to Alzheimer’s disease. TRAILBLAZER-ALZ 3 will evaluate whether treatment with donanemab can slow the clinical progression of Alzheimer’s disease in trial participants. vice president of pain and neurodegeneration, Lilly.

Drug Target Review
OCTOBER 5, 2023
This study sought to shed light on the safety and effectiveness of aHSCT in routine healthcare settings, moving beyond the confines of clinical trials. The research team identified 231 individuals diagnosed with relapsing-remitting MS, with 174 of them having undergone aHSCT treatment before the year 2020.

The Pharma Data
OCTOBER 12, 2021
Trial met primary endpoint. The trial met the primary endpoint, with a cure of 600 mg of AZD7442 given by intramuscular (IM) injection reducing the threat of developing severe COVID-19 or death (from any cause) by 50 compared to placebo in rehabilitants who had been characteristic for seven days or lower.

The Pharma Data
OCTOBER 25, 2021
First immunotherapy combination to demonstrate superior clinical outcomes over standard of care in a global, randomised trial in this setting. 4 In December 2020, Imfinzi was granted Orphan Drug Designation in the US for the treatment of BTC.

The Pharma Data
FEBRUARY 21, 2022
All patients in the trial received a HER2 test, and the results were centrally confirmed. The trial met the key secondary endpoint of PFS in patients with HER2-low metastatic breast cancer regardless of HR status (HR-positive or HR-negative). For more information about the trial, visit ClinicalTrials.gov.

The Pharma Data
NOVEMBER 18, 2021
Six-months follow-up of prevention trial showed 83% reduced risk of symptomatic COVID-19, with no severe disease or deaths with AZD7442. Separate treatment trial showed 88% reduced risk of severe COVID-19 or death when treated within three days of symptom onset.

FDA Law Blog: Biosimilars
JANUARY 15, 2024
FDA complied with that order, later extended the deadline because of COVID, and eventually settled on a PMTA deadline of September 9, 2020. FDA said randomized clinical trials could be used, but so could observational studies with respect to cessation data. FDA also directed manufacturers to produce detailed marketing plans.

LifeSciVC
JANUARY 16, 2024
Whether trial design, execution, or otherwise, drug development even where there is precedent is a challenging road and should not be taken for granted. Additional trials (e.g., The incretins as a class also look interesting across a number of follow-on indications, as evidenced by clinical trials in these areas (below).

Drug Target Review
JULY 6, 2023
million scientific procedures involving live animals were carried out in 2020. 1 Ethical concerns surrounding the use of animal studies is increasing, especially considering 90 percent of drug candidates fail in clinical trials. million scientific procedures involving live animals were carried out in 2020. In Britain, 2.88

FDA Law Blog: Drug Discovery
DECEMBER 7, 2022
The gene therapy demonstrated that it increased Factor IX (“FIX”) plasma levels at 6 months, the original primary endpoint of the Phase 3 trial. Again, this was despite the fact that subjects in the trial had demonstrated durable FIX activity to this point, with a mean of 41.5%

The Pharma Data
APRIL 8, 2022
NYSE: PFE) announced updated results from the Phase 3 CROWN trial, which evaluated LORBRENA® (lorlatinib, available in Europe under the brand name LORVIQUA) versus XALKORI® (crizotinib) in people with previously untreated anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC). Pfizer Inc.

New Drug Approvals
SEPTEMBER 29, 2024
Both were reversed by treatment with COR388 ( 2020 AAIC abstract ). Findings Two Phase 1 trials of atuzaginstat were completed by June 2019. Gingipains also were reported to degrade ApoE, and 28 days of treatment with COR388 was claimed to reduce CSF ApoE fragments ( 2020 AAIC abstract ). This trial involves 93 sites in the U.S.

Broad Institute
OCTOBER 4, 2023
A new small-molecule drug candidate being tested in an early-stage clinical trial aims to improve patient responses to immunotherapy. AbbVie and Calico are currently testing the molecule and another related molecule , also developed by AbbVie and Calico, in phase 1 clinical trials. We were really impressed by that.”

The Pharma Data
NOVEMBER 8, 2021
Bakris, MD, Department of Medicine, American Heart Association Comprehensive Hypertension Center, University of Chicago Medicine, USA and top investigator of the FIDELIO-DKD trial. “ In financial 2020, the Group employed around people and had deals of41.4 The Bayer brand stands for trust, trustability and quality throughout the world.

Codon
JULY 14, 2024
However, the subsequent death of another patient thrust Denys into a contentious trial. Despite the high costs, researchers in Kyoto generated enough cultured platelets in 2022 to transfuse a 55-year-old woman for an initial human trial. The results of the trial are expected at the end of 2024. Data from Rousseau G.F.

The Pharma Data
MAY 16, 2021
The Phase 3 trial is expected to enrol more than 35,000 adult participants from a broad range of countries and will assess the efficacy of two vaccine formulations including the D614 (Wuhan) and B.1.351 1.351 (South African) variants. An earlier stage collaboration with SK Bioscience is also ongoing. Source link.

DrugBank
JULY 18, 2024
It commences with preclinical studies in animal models to assess safety and immunogenicity, followed by three phases of human clinical trials. Phase IV trials, also known as post-market surveillance studies, continue to monitor the vaccine's safety and effectiveness in the real world.

Vial
APRIL 10, 2024
With over 1,100 CROs worldwide as of 2020, choosing the appropriate partner often requires evaluating the advantages and disadvantages of large global CROs versus mid/small ones. Still in its early stages, Vial was established in 2020 and has quickly emerged as a leading CRO. Vial stands out from IQVIA in several key aspects.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content