Four Crucial Questions about the Humira Biosimilar Price War (rerun)
Drug Channels
OCTOBER 3, 2023
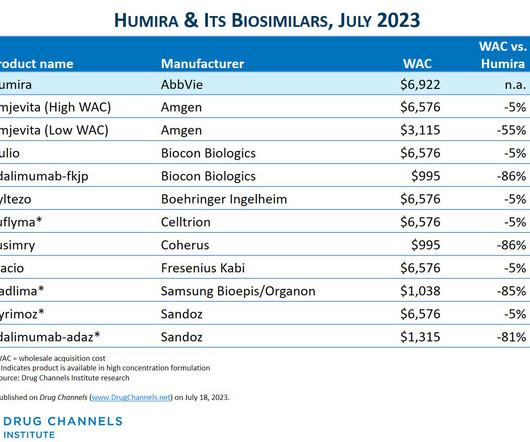
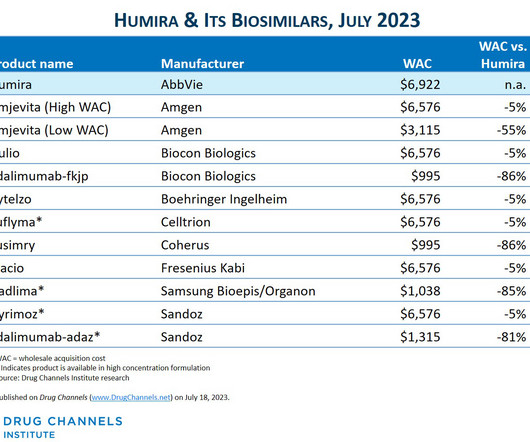
Since I published the article below in July 2023 , there have been three notable market develpoments: IQVIA has reported that as as of mid-2023, there was almost no adoption of Amgen's Amjevita, the first Humira biosimilar. Boehringer-Ingelheim launched an unbranded, low WAC version of its interchangeable biosimilar.












































Let's personalize your content