Drug discovery informatics market predicted to grow 12.6% by 2028
Drug Discovery World
FEBRUARY 20, 2024
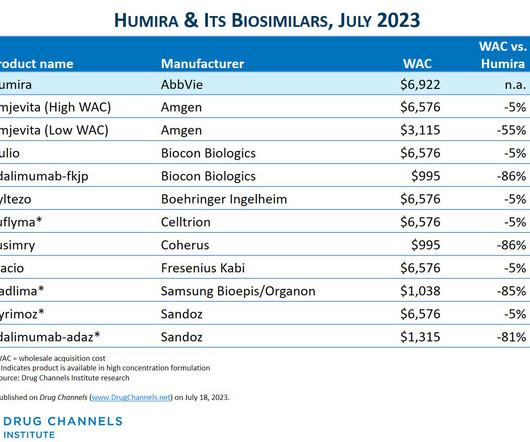
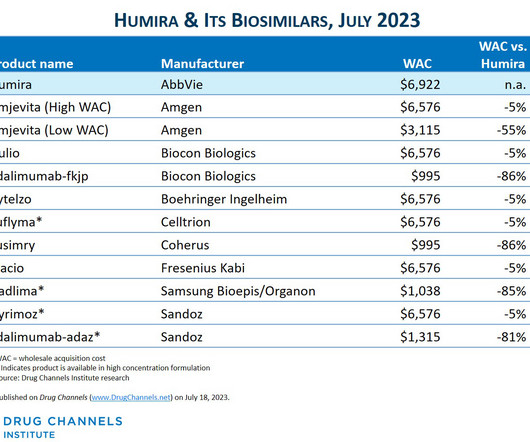
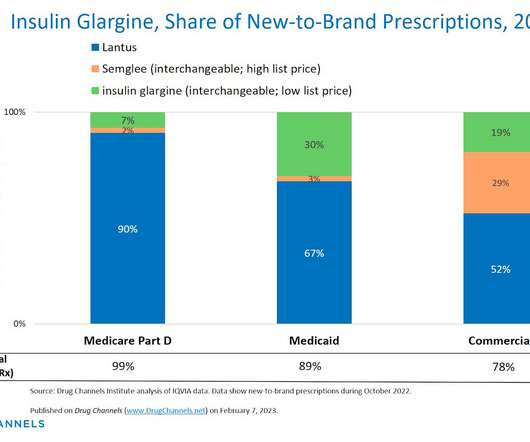
The drug discovery informatics market is predicted to grow from $3.77 The new report says the anticipated growth to 2028 will be fuelled by the expansion of precision medicine, advancements in drug-drug interaction prediction, analysis of patient-derived data, development of biosimilars, and the increasing number of drug candidates.











































Let's personalize your content