Four Crucial Questions about the Humira Biosimilar Price War (rerun)
Drug Channels
OCTOBER 3, 2023
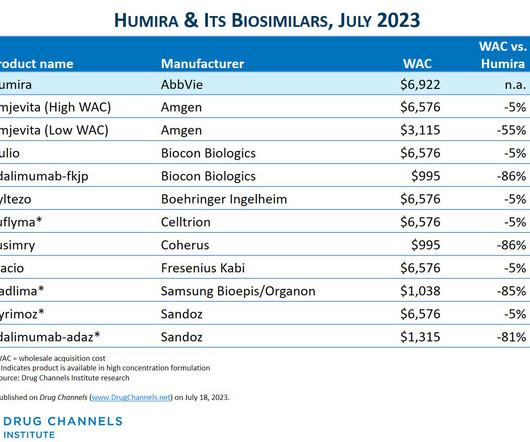
This week, I’m rerunning some popular posts while I put the finishing touches on our new 2023-24 Economic Report on Pharmaceutical Wholesalers and Specialty Distributors. CVS Health launched Cordavis, a new subsidiary that will market a private label, low-list-price version of Sandoz’ Hyrimoz. The Humira biosimilar market has arrived!
















































Let's personalize your content