Novavax Announces COVID-19 Vaccine Clinical Development Progress
The Pharma Data
NOVEMBER 30, 2020
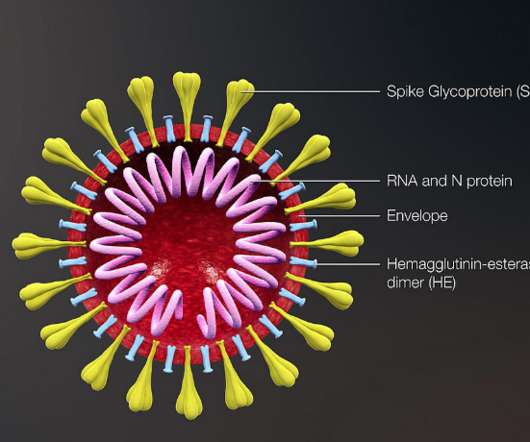
Two of the three planned late-stage efficacy trials for NVX-CoV2373 sponsored by Novavax are fully enrolled, and more than 20,000 participants have been dosed to-date. In alignment with Novavax’ commitment to transparency, Phase 3 clinical trial protocols are posted to the company’s website at Novavax.com/resources upon finalization.












































Let's personalize your content