Antibody treatment for geographic atrophy enters clinical trials
Drug Discovery World
OCTOBER 11, 2023
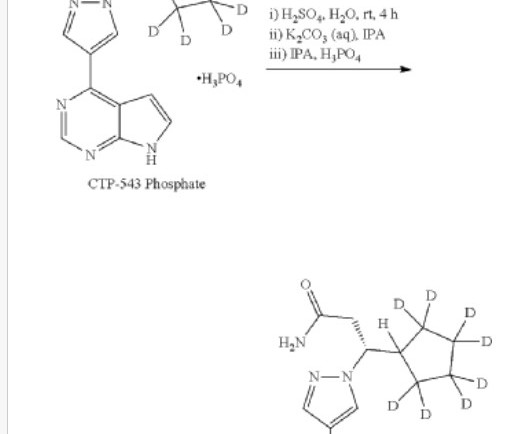
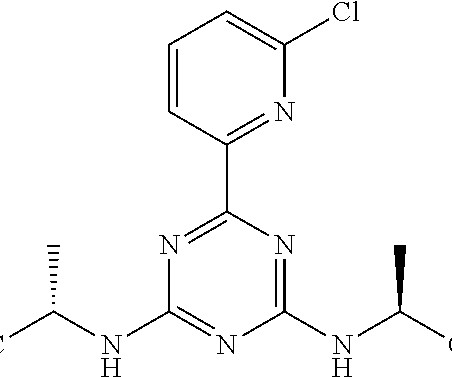
Collaborators Boehringer Ingelheim and CDR-Life have commenced a Phase I evaluation of BI 771716, their antibody fragment-based treatment developed to preserve the vision of people with geographic atrophy (GA). GA is an irreversible retinal disease that occurs in people with late-stage dry age-related macular degeneration (AMD).










































Let's personalize your content