Breaking C-F bonds in drugs
Metabolite Tales Blog
MAY 12, 2023
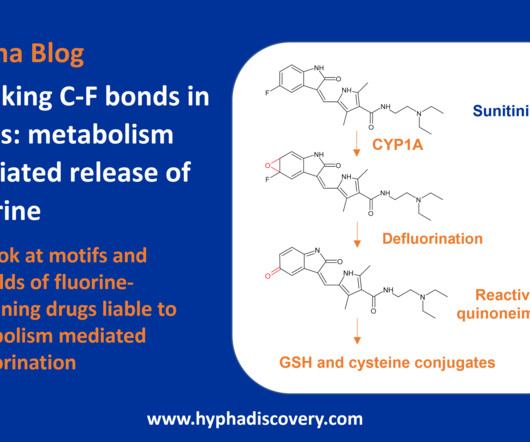
Breaking C-F bonds in drugs – metabolism mediated release of fluorine By Samuel Coe and Julia Shanu-Wilson Lenacapavir, recently approved for multi-drug resistant HIV-1 infection, contains 10 fluorine atoms. Increasingly used in drug design, some drug structures are now bristling with fluorine atoms.









Let's personalize your content