FDA Approved Oncology Drugs 2023
Crown Bioscience
JUNE 6, 2024
This marks the second-highest count in the past 30 years, with the highest being 59 new drug approvals in 2018 and represents an impressive 50% increase in drugs approved in 2022.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Crown Bioscience
JUNE 6, 2024
This marks the second-highest count in the past 30 years, with the highest being 59 new drug approvals in 2018 and represents an impressive 50% increase in drugs approved in 2022.
Metabolite Tales Blog
JANUARY 26, 2023
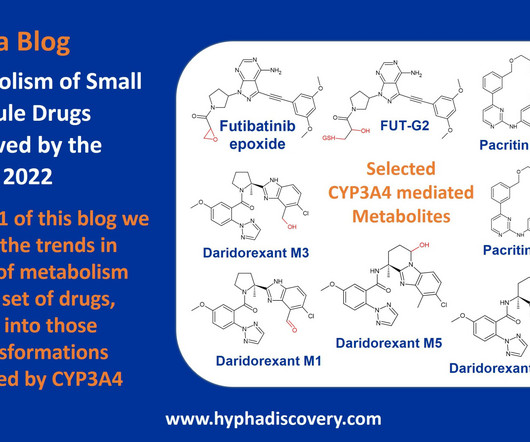
Metabolism of 2022 FDA approved small molecule drugs – Part 1 Does CYP3A4 still rule? By Julia Shanu-Wilson It won’t come as much surprise to learn that of the 17 small molecules* approved by the FDA in 2022, CYP3A4 was the major player in drug metabolism. 2022; 131(5): 311- 324. Tang et al.,
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Metabolite Tales Blog
APRIL 4, 2023
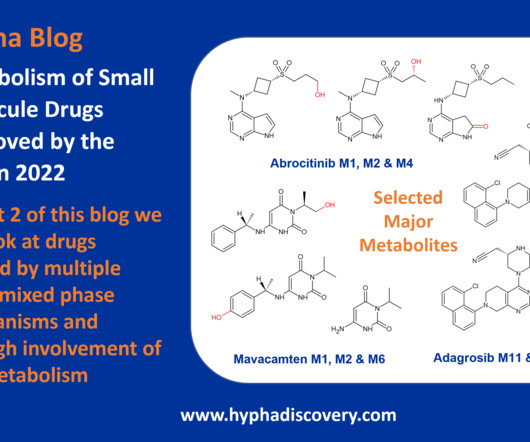
Metabolism of 2022 FDA approved small molecule drugs part 2 Mixing it Up By Julia Shanu-Wilson In Part 1 of this topic we looked at metabolism of the small molecule drugs approved by the FDA in 2022 that were mediated by CYP3A4. Poster at ISSX/MDO 2022 Seattle meeting. [6] Br J Pharmacol.

The Pharma Data
JUNE 2, 2023
Food and Drug Administration (FDA) for the treatment of adult patients with deleterious or suspected deleterious BRCA -mutated ( BRCA m) metastatic castration-resistant prostate cancer (mCRPC). Patients should be selected for therapy based on an FDA-approved companion diagnostic for LYNPARZA. In the U.S., For the U.S.

The Pharma Data
FEBRUARY 25, 2022
Several FDA-approved drugs – including for type 2 diabetes, hepatitis C and HIV – significantly reduce the ability of the Delta variant of SARS-CoV-2 to replicate in human cells, according to new research led by scientists at Penn State. Commun Biol 5, 169, 2022. ” Other authors on the paper include Sydney A.
Metabolite Tales Blog
JANUARY 23, 2024
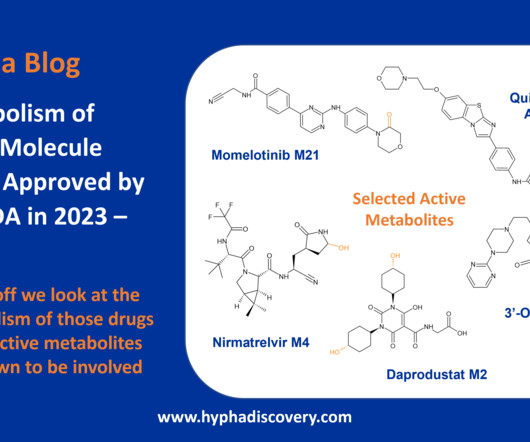
Metabolism of 2023 FDA Approved Small Molecules – PART 1 By Julia Shanu-Wilson 2023 was a fruitful year for drug approvals by the FDA, with a crop of 34 small molecules out of a total of 55 new drugs [1]. References [1] 2023 Novel Small Molecule FDA Drug Approvals. 2] Iversen et al., 131(5): 311- 324.

Eye on FDA
JANUARY 16, 2023
By contrast in 2021 there were only 10 (see blue bars below in the chart tracking meetings from 2017 – 2022). Yet in the midst of that range, the number of new molecular entities approved by FDA actually increased. But conversely, in 2022 the number of NMEs plummeted. Pre-pandemic numbers were consistently higher.

The Pharma Data
APRIL 19, 2022
Food and Drug Administration (FDA) has approved commercial production at the company’s new CAR T-cell therapy manufacturing facility in Frederick, Maryland. The site will produce Kite’s FDA approved CAR T-cell therapy used to treat blood cancer. Our median cycle time is industry leading at 16 days in the U.S.,

The Pharma Data
FEBRUARY 24, 2022
Allergan, an AbbVie (NYSE: ABBV) company, today announced new data to be presented from Allergan’s leading portfolio of eye care treatments at the 2022 American Glaucoma Society (AGS) Annual Meeting being held in Nashville, TN and virtually from March 3-6. The DURYSTA data presentations coincide with the two-year anniversary of the U.S.

FDA Law Blog: Drug Discovery
DECEMBER 7, 2022
Valentine — On November 22, 2022, FDA approved CSL Behring’s BLA for Hemgenix (etranacogene dezaparvovec), an AAV-based gene therapy for the treatment of adults with Hemophilia B who currently use Factor IX prophylaxis therapy, have current or historical life-threatening hemorrhage, or have repeated, serious spontaneous bleeding episodes.

Fierce BioTech
OCTOBER 5, 2023
Discover Strategies for Combatting Disruptions in Gene Therapy Development Cell and gene therapy development has exploded, with Q4 2022 showing more FDA approvals than over the past five years combined.[1] 1] How can sponsors keep pace with this rapidly growing market and avoid costly delays that can threaten critical timelines?

New Drug Approvals
DECEMBER 19, 2022
FDA 12/1/2022, To treat adults with relapsed or refractory acute myeloid leukemia with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation, Rezlidhia Olutasidenib , sold under the brand name Rezlidhia , is an anticancer medication used to treat relapsed or refractory acute myeloid leukemia. [1] 1 December 2022. J Med Chem.

FDA Law Blog: Biosimilars
MARCH 29, 2023
While many states have had standing orders that allow for the dispensing of naloxone without an individual prescription, “harm reduction programs” that provide products and services to at-risk individuals still faced logistical difficulties in acquiring naloxone due to its prescription-only status which I discussed in this October 2022 blog post.

FDA Law Blog: Biosimilars
APRIL 21, 2024
But that’s the controversy here: Did FDA approve LYTGOBI NDA 214801 on September 30, 2022 when the Agency issued its initial approval letter , or on October 5, 2022 when FDA issued a corrected approval letter ? That’s important, because PTE applications for U.S. Patent Nos. Patent Nos.

The Pharma Data
APRIL 7, 2022
This unprecedented CMS decision effectively denies all Medicare beneficiaries access to ADUHELM ® (aducanumab-avwa), the first and only FDA-approved therapy in a new class of Alzheimer’s drugs. It may also limit coverage for any future approved treatment in the class. About Biogen.

The Pharma Data
FEBRUARY 12, 2022
This data will be presented at the 29th Conference on Retroviruses and Opportunistic Infections (virtual CROI 2022) taking place from February 12-16. Veklury was approved by the FDA on October 22, 2020 for grown-ups and pediatric cases 12 times of age and aged and importing at least 40 kg for the treatment of COVID-19 taking hospitalization.

The Pharma Data
MARCH 31, 2023
Drug overdose persists as a major public health issue in the United States, with more than 101,750 reported fatal overdoses occurring in the 12-month period ending in October 2022, primarily driven by synthetic opioids like illicit fentanyl. “The FDA remains committed to addressing the evolving complexities of the overdose crisis.
Drug Target Review
APRIL 12, 2024
This holds particular significance, as it is a prerequisite for FDA approval in biotechnology that any cell clones must originate from a single-cell progenitor. In 2022, SEED Biosciences received its very first and prestigious New Product Award. He was awarded with the Ignite Award at SLAS 2019.

EG Life Sciences
FEBRUARY 15, 2023
The year 2022 reflected a transformative path for the drug development industry. It is without a doubt that 2022 predicted change and opportunity in biopharma and biotech clinical trials in 2023. The US Food and Drug Administration (FDA) approved around 26 novel drugs in 2022.
Drug Target Review
APRIL 12, 2024
This holds particular significance, as it is a prerequisite for FDA approval in biotechnology that any cell clones must originate from a single-cell progenitor. In 2022, SEED Biosciences received its very first and prestigious New Product Award. He was awarded with the Ignite Award at SLAS 2019.

The Pharma Data
OCTOBER 9, 2021
FDA is anticipated in late 2022. “As With the limited availability of pediatric patients for clinical trial inclusion, researchers can extrapolate data from trials with adults to determine the potential efficacy and tolerability of a treatment for a pediatric population. A decision from the U.S.
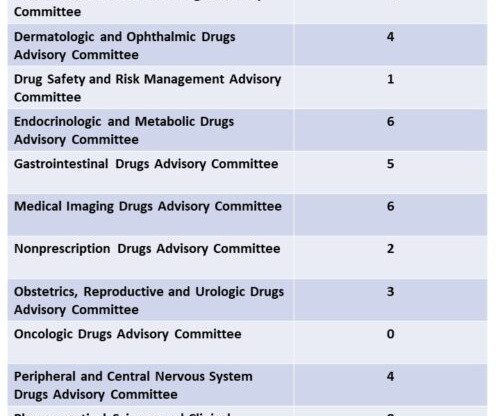
Eye on FDA
DECEMBER 6, 2023
For those working closely with the development of new medicines for FDA approval, it can be informative respecting the future to look back at recent activity and take note of any potential changes from years past. By contrast, in 2023, the number of recommendations for approval was over three times the number of negative outcomes.

New Drug Approvals
JANUARY 3, 2023
Adagrasib Formula C32H35ClFN7O2 cas 2326521-71-3 Mol weight 604.1174 Antineoplastic Disease Non-small cell lung cancer 2022/12/12 FDA APPROVED, KRAZATI (Mirati Therapeutics) MRTX-849 MRTX849 KRAS G12C inhibitor MRTX849 Adagrasib , sold under the brand name Krazati , is an anticancer medication used to treat non-small cell lung cancer. [1]

The Pharma Data
OCTOBER 22, 2021
With 25 request share in soybean pesticides in Brazil, Bayer plans to upgrade this product in 2022 with the launch of Fox Supra, further expanding its commanding position in this largely important request, with a peak deals eventuality of further than€ 500 million. is underway.
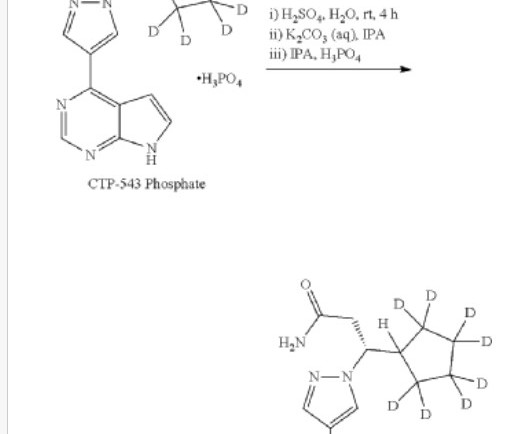
New Drug Approvals
SEPTEMBER 10, 2024
Deuruxolitinib C 17 H 18 N 6 , 314.422 Fda approved Leqselvi , 7/25/2024, To treat severe alopecia areata C-21543, CTP 543, CTP-543, CTP543 (3r)-3-(2,2,3,3,4,4,5,5-d8)cyclopentyl-3-(4-(7h-pyrrolo(2,3-d)pyrimidin-4-yl)-1h-pyrazol-1-yl)propanenitrile 1h-pyrazole-1-propanenitrile,beta.-(cyclopentyl-2,2,3,3,4,4,5,5-d8)-4-(7h-pyrrolo(2,3-d)pyrimidin-4-yl)-,

The Pharma Data
MARCH 28, 2022
The FDA approval is based on the results from the SUSTAIN FORTE trial. We are pleased with the FDA approval for a higher 2.0 The approval of the 2.0 mg in the United States in the second quarter of 2022. mg is now approved in the US, the EU, Canada and Switzerland. Compared to semaglutide 1.0
Drug Target Review
JUNE 5, 2023
As such, our team remains on track to complete our 5×5 strategy – laid out in October 2022 – by harnessing our technology and resources to continue efficiently growing Zymeworks’ pipeline of in-house and partnered candidates. Author Bio: Paul Moore, PhD Dr Moore joined Zymeworks in July 2022 as Chief Scientific Officer.

FDA Law Blog: Biosimilars
OCTOBER 1, 2024
FOOD AND DRUG ADMINISTRATION et al Challenge to FDA approval of generic Hetlioz (tasimelteon) Pending 1:2023cv00629 (COFC) VANDA PHARMACEUTICALS, INC. FOOD AND DRUG ADMINISTRATION et al Challenge to FDA approval of generic Hetlioz (tasimelteon) Pending ( Motion to Dismiss Denied-in Part/Granted-in-Part ) 1:2023cv02884 (D.D.C.)

The Pharma Data
JUNE 2, 2022
BYOOVIZ™ is the first FDA approved ophthalmology biosimilar BYOOVIZ, priced 40% lower than LUCENTIS®, provides an equally effective and more affordable treatment option to patients suffering from retinal disorders BYOOVIZ will be commercially available through major distributors across the U.S. on July 1, 2022. Biogen Inc.

FDA Law Blog: Biosimilars
FEBRUARY 27, 2024
156, a patent may be extended only once (even if it would be eligible for extension on more than one occasion because it applies to several FDA-approved products), and only one patent may be extended for each regulatory review period. Two PTE applications were submitted to FDA ( See FDA Docket Nos. Patent Nos.

Codon
JULY 14, 2024
In January 2022, the American Red Cross declared its first-ever national blood crisis owing to a severe blood shortage—the worst shortfall in more than a decade. Despite the high costs, researchers in Kyoto generated enough cultured platelets in 2022 to transfuse a 55-year-old woman for an initial human trial.

Broad Institute
APRIL 25, 2024
The Sentinel team put an early version of their system to the test in 2020 to respond to COVID-19 and again in 2022 for Mpox surveillance and response efforts. Sentinel is also developing a SHINE test to detect Lassa virus in rural clinics in Nigeria, where there are currently no FDA-approved diagnostics for the disease.

The Pharma Data
FEBRUARY 25, 2022
Food and Drug Administration (FDA) has accepted for review the Prior Approval Supplement (PAS) to the Biologics License Application (BLA) for ABRILADA™ (adalimumab-afzb) as an interchangeable biosimilar to Humira® (adalimumab). The Biosimilar User Fee Act (BsUFA) goal date for an FDA decision is in Q4 2022. “An

The Pharma Data
SEPTEMBER 8, 2021
Adds Rezurock™ (belumosudil) an FDA-approved, first-in-class treatment for adult and pediatric patients 12 years and older with chronic graft-versus-host disease (cGVHD) after failure of at least two prior lines of systemic therapy. The transaction is expected to be modestly dilutive to Sanofi’s EPS in 2022. Transaction Terms.

FDA Law Blog: Biosimilars
AUGUST 12, 2024
The NADA applicant requested reconsideration, but FDA refused to set the testing phase at the INAD opening and instead used the date that a major health or environmental effects test on the drug was initiated. Plaintiffs performed the necessary studies on BRAVECTO and filed an NADA on April 8, 2014; FDA approved the NADA on May 15, 2014.

The Pharma Data
APRIL 7, 2023
Food and Drug Administration (FDA) has accepted for review the Supplemental New Drug Applications (sNDAs) for BRAFTOVI® (encorafenib) + MEKTOVI® (binimetinib) for patients with metastatic non-small cell lung cancer (NSCLC) with a BRAF V600E mutation, as detected by an FDA-approved test. Updated February 14, 2022.

The Pharma Data
APRIL 15, 2022
In January 2022, Sierra Oncology announced positive topline results from the MOMENTUM phase III trial. New GSK reaffirms its full-year 2022 guidance, the medium-term outlook for 2021-2026 of more than 5% sales and 10% adjusted operating profit CAGR* at CER**, and long-term sales ambition.

Advarra
AUGUST 16, 2022
Food and Drug Administration (FDA) approval. Since then, the FDA has significantly changed its approach to rare and orphan diseases. The FDA Since 1983. The Orphan Drug Act of 1983 was instrumental in changing the number of orphan drugs approved in the U.S. FDA Expedited Programs.

The Pharma Data
NOVEMBER 3, 2020
mg, effective January 1, 2022. Prior to the January 1, 2022 effective date of the newly issued Category I CPT code, payments to physicians are expected to continue with the service reported using CPT code 0356T. DEXTENZA is FDA approved for the treatment of ocular inflammation and pain following ophthalmic surgery.

Broad Institute
JULY 26, 2022
Resources, services, and tools By Maria Nemchuk July 26, 2022 Breadcrumb Home Resources, services, and tools Key scientific datasets and computational tools developed by our scientists and their collaborators. Learn more ENCODE Integrated, annotated encyclopedia of functional and regulatory elements in the genome.

Alta Sciences
APRIL 17, 2024
According to a 2022 article published in Molecular Psychiatry, treatment resistance affects 20 to 60% of patients with psychiatric disorders. According to an article published in Frontiers in Pharmacology , as of October 2023, there were 15 FDA-approved ADCs on the market, and more than one hundred in clinical development in the U.S.A.,

Drug Target Review
JUNE 21, 2023
Human genetics evidence supports two-thirds of the 2021 FDA-approved drugs. 2022 Aug;21(8):551. 2022 Jul 8;23(14):7570. 2022 Mar;603(7899):124-130. Epub 2022 Feb 23. DOI: 10.1101/2023.02.07.23285407. Ochoa D, Karim M, Ghoussaini M, Hulcoop DG, McDonagh EM, Dunham I. Nat Rev Drug Discov. PMID: 35804044.

KIF1A
APRIL 25, 2024
September 2022 – We were contacted by n-Lorem to obtain blood from Sloane, my husband, and me. However, we needed to wait for Susannah to finish her trial before we could submit to the FDA for Sloane’s ASO use. February 2024 – n-Lorem submitted an IND (investigational new drug) to the FDA for Sloane.

Advarra
NOVEMBER 17, 2022
In 2016, the Food and Drug Administration (FDA) approved Spinraza (nusinersen). While the FDA’s approval of nusinersen may not seem extraordinary, it was. Nusinersen’s approval marked the first time nonclinical data supported conducting initial clinical trials involving children. o) define children. [2]
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content