The Competitive Edge of Biosimilars
DrugBank
SEPTEMBER 26, 2024
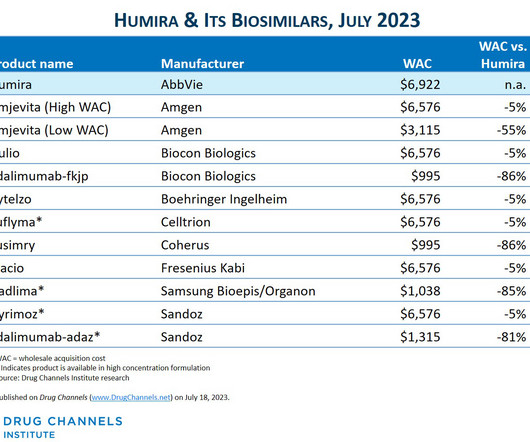
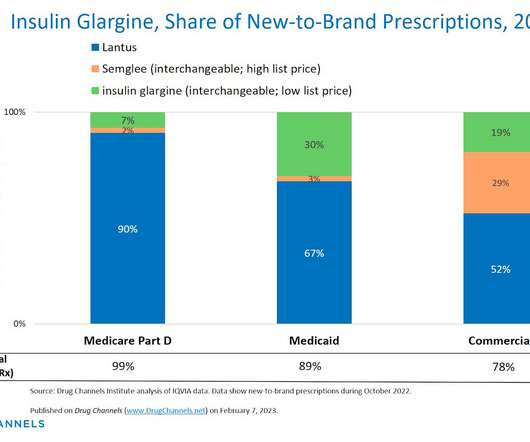
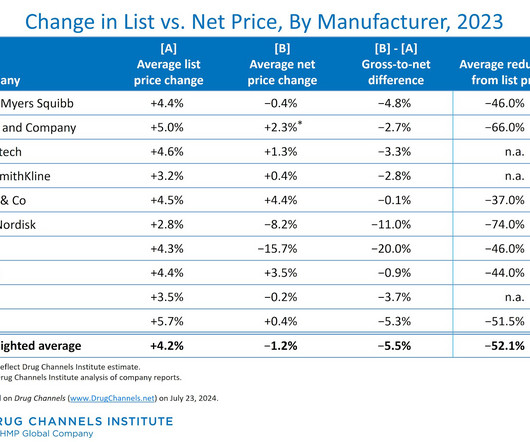
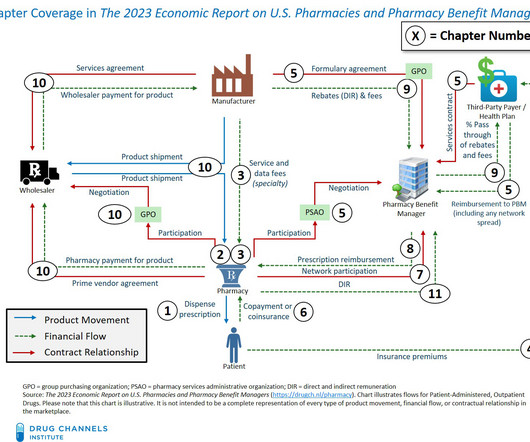
Biosimilars Biosimilars, while highly similar to their reference biopharmaceuticals, offer distinct advantages that position them as preferred therapeutic options in many cases. This is because biosimilars are not new drugs but highly similar versions of already approved therapies with established safety and efficacy profiles.










































Let's personalize your content