Are Gaps in Your Clinical Pharmacology Program Jeopardizing Your Drug’s Approval?
Fierce BioTech
OCTOBER 26, 2023
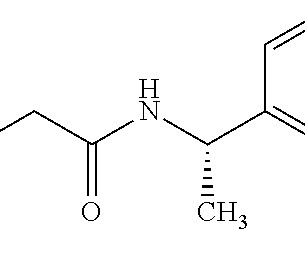
Are Gaps in Your Clinical Pharmacology Program Jeopardizing Your Drug’s Approval? pesurya Thu, 10/26/2023 - 14:05 Tue, 12/05/2023 - 11:00 Resource Type Webinar Julie Bullock, PharmD Krithika Shetty, PhD Blaire Osborn, Ph.D. png Listing Introduction Clinical pharmacology information comprises more than 50% of a drug label.











































Let's personalize your content