FDA Returns Disappointing News for ALS Stem Cell Therapy
PLOS: DNA Science
OCTOBER 19, 2023
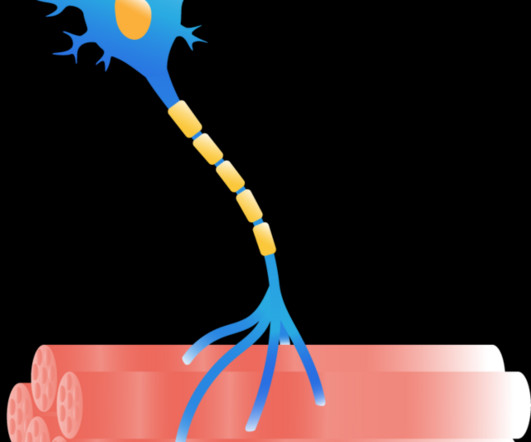
Remove MSCs from a person with ALS, stimulate them to release the correct brew of neurotrophic factors, then return the doctored cells into the patients spinal fluid, where they should bathe neurons and their supportive glial cells with the nurturing factors.



















Let's personalize your content