FDA Approved Oncology Drugs 2023
Crown Bioscience
JUNE 6, 2024
In a significant development, the US Food and Drug Administration’s (FDA) Center for Drug Evaluation and Research (CDER) approved 55 new drugs in 2023.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
Crown Bioscience
JUNE 6, 2024
In a significant development, the US Food and Drug Administration’s (FDA) Center for Drug Evaluation and Research (CDER) approved 55 new drugs in 2023.

Drug Discovery World
JUNE 23, 2023
EMBARK, the global, randomised, double-blind, placebo-controlled Phase III trial for Elevidys, will serve as the post-marketing confirmatory trial and is fully enrolled with top-line results expected in late 2023. The post FDA approves first gene therapy for Duchenne muscular dystrophy appeared first on Drug Discovery World (DDW).
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Discovery World
DECEMBER 5, 2023
Jaypirca is the first FDA-approved non-covalent (reversible) BTK inhibitor. This is the marketing authorisation approval for the drug in 2023, after it was accelerated approval by the FDA for the treatment of mantle cell lymphoma (MCL) in January.

Drug Discovery World
JUNE 6, 2023
Today’s FDA approval of Abrysvo recognises significant scientific progress, and importantly helps provide older adults potential protection against RSV and an opportunity to improve community health by helping prevent the disease.”
Drug Discovery World
AUGUST 14, 2023
Janssen’s Akeega (niraparib and abiraterone acetate) is now available in the US for adult patients with deleterious or suspected deleterious BRCA-positive metastatic castration-resistant prostate cancer (mCRPC) following FDA approval. The European Commission (EC) granted marketing authorisation for Akeega in April 2023.

Drug Discovery World
AUGUST 21, 2023
The FDA approval is based on data from the Phase III MOVE trial, which demonstrated that palovarotene effectively reduced annualised heterotopic ossification (HO) volume compared with no treatment beyond standard of care (54% reduction with weighted linear mixed effect model).

Drug Discovery World
AUGUST 23, 2023
Pfizer’s Abrysvo (Respiratory Syncytial Virus Vaccine) was approved for use at 32-36 weeks gestational age of pregnancy to prevent lower respiratory tract disease (LRTD) and severe LRTD caused by RSV in infants from birth to six months of age.

Drug Discovery World
DECEMBER 21, 2023
DDW’s top 10 most popular articles for 2023 reflect the key subjects in drug discovery and development. In a record year for gene therapy approvals, it isn’t surprising that cell & gene therapy (CGT) has been a hot topic. DDW’s Senior Digital Content Editor Diana Spencer reviews the year’s top ten articles.

Drug Discovery World
JUNE 12, 2023
The US Food and Drug Administration (FDA) has approved Minoryx Therapeutics’ Phase III clinical trial (CALYX) of leriglitazone, to treat adult male X-linked Adrenoleukodystrophy (X-ALD) patients with cerebral Adrenoleukodystrophy (cALD).

Drug Discovery World
MAY 15, 2023
CytoAgents anticipates the clinical trial to launch at its first trial site in the summer of 2023. The post FDA approves study into CAR-T associated cytokine storm appeared first on Drug Discovery World (DDW).

Drug Discovery World
DECEMBER 27, 2023
In other news, the FDA approved a promising antibody-drug conjugate for bladder cancer and trial results demonstrated that psilocybin could reduce depression symptoms in cancer patients. The post DDW Highlights: 27 December 2023 appeared first on Drug Discovery World (DDW).

Drugs.com
AUGUST 22, 2024
22, 2024 -- Updated shots to shield against COVID-19 infection were approved by the U.S. Food and Drug Administration on Thursday.This year's approval for the updated mRNA vaccines comes much sooner than happened in 2023, when fall. THURSDAY, Aug.

Drug Discovery World
DECEMBER 27, 2023
In other news, the FDA approved a promising antibody-drug conjugate for bladder cancer and trial results demonstrated that psilocybin could reduce depression symptoms in cancer patients. The post New DDW Highlights podcast for 27 December 2023 appeared first on Drug Discovery World (DDW).
Drug Discovery World
JUNE 19, 2023
Regulatory approvals and industry guidelines are key to the continued development and discovery of drugs, which is a common theme throughout this week’s news highlights, including an FDA approval for Pfizer, a NICE recommendation for AstraZeneca UK, and an update to the HER2 Breast Cancer Testing Guidelines.

Drug Discovery World
JULY 10, 2023
In other headline news, the FDA approved the first cellular therapy for type 1 diabetes which could allow some patients to become insulin-independent, and a new study linked severe Covid-19 outcomes with high levels of antibiotics use. The post DDW Highlights: 10 July 2023 appeared first on Drug Discovery World (DDW).

Drug Discovery World
JULY 10, 2023
In other headline news, the FDA approved the first cellular therapy for type 1 diabetes which could allow some patients to become insulin-independent, and a new study linked severe Covid-19 outcomes with high levels of antibiotics use. The post New DDW Highlights podcast: 10 July 2023 appeared first on Drug Discovery World (DDW).

Drug Discovery World
SEPTEMBER 14, 2023
As the second quarter of 2023 draws to a close, DDW’s Megan Thomas reviews key updates and results from three top pharmaceutical companies. Business updates Pfizer reported second quarter 2023 revenues totalling $12.7 billion, a decrease of $15.0 at CER and business EPS growth of 8.1% Specialty care grew 11.8% Vaccines were up 9.1%

Drug Discovery World
AUGUST 14, 2023
Emeritus Professor Ian Frazer, University of Queensland and Director of Microba, has been announced as the Millis Oration keynote speaker for AusBiotech 2023. The title of his presentation will be ‘Navigating the future: Predictions and potential of Australian biotechnology’.

Drug Discovery World
OCTOBER 10, 2023
A new class of antidepressants In July 2023, AlzeCure Pharma published the results of a preclinical study supporting the antidepressive effects ACD856, the first in a new class of antidepressants. Over recent months, there have been a number of ground-breaking discoveries in drug discovery for mental health conditions.

Drugs.com
SEPTEMBER 11, 2023
11, 2023 -- The U.S. MONDAY, Sept. Food and Drug Administration on Monday gave the green light to new COVID boosters for Americans, setting the stage for the updated vaccines to become available within days. The COVID-19 shots from Pfizer and.
Drugs.com
DECEMBER 8, 2023
8, 2023 -- The U.S. Food and Drug Administration on Friday approved two milestone gene therapies for sickle cell disease, including the first treatment ever approved that uses gene-editing technology.Casgevy, developed by Vertex. FRIDAY, Dec.
Drug Discovery World
AUGUST 9, 2023
The new acquisition gives Biogen rights to Reata’s portfolio of products for neurological diseases, including the FDA-approved Skyclarys (omaveloxolone), the only approved treatment for Friedreich’s ataxia (FA) in the United States.

Drugs.com
AUGUST 30, 2023
August 28, 2023 -- FDA has approved several first generics of Vyvanse (lisdexamfetamine dimesylate) capsules and chewable tablets for attention-deficit/hyperactivity disorder (ADHD) in patients six years and older and moderate to severe binge-eating.
Drugs.com
DECEMBER 8, 2023
8, 2023 -- The U.S. Food and Drug Administration on Friday approved two milestone gene therapies for sickle cell disease, including the first treatment ever approved that uses gene-editing technology. FRIDAY, Dec. Casgevy, developed by Vertex.

PLOS: DNA Science
MARCH 28, 2024
Results from the study that led to the FDA approval appeared in The Lancet Neurology in April 2024 with commentary. In 2023, two gene-based treatments became available. The post FDA Approves Duvystat, New Oral Treatment for Duchenne Muscular Dystrophy (DMD) appeared first on DNA Science.
Drugs.com
NOVEMBER 17, 2023
16, 2023 -- The first home test for chlamydia and gonorrhea will soon hit the market, following its approval Wednesday by the U.S. THURSDAY, Nov. Food and Drug Administration. People will be able to buy the Simple 2 Test over-the-counter at a.

Drugs.com
JUNE 14, 2023
WEDNESDAY, June 14, 2023 -- The U.S. Food and Drug Administration approved Linzess (linaclotide) as a once-daily treatment for pediatric patients ages 6 to 17 years with functional constipation. The approval was based on data from 328 participants.

SCIENMAG: Medicine & Health
JULY 26, 2023
July 26, 2023 – Octapharma USA today announced that Balfaxar® (prothrombin complex concentrate, human-lans; marketed in Europe and Canada as octaplex®) has received U.S. PARAMUS, N.J.,

Drugs.com
JULY 5, 2023
WEDNESDAY, July 5, 2023 -- A new blood test approved by the U.S. Food and Drug Administration can predict imminent preeclampsia, helping pregnant women who are at risk for this severe and sometimes deadly form of high blood pressure. The test can.
Drugs.com
SEPTEMBER 7, 2023
7, 2023 -- New COVID-19 booster shots could soon pass the needed hurdles for vaccinations to begin next week. Food and Drug Administration plans say boosters could be approved as soon as Friday, NBC News. THURSDAY, Sept. Sources familiar with U.S.

Drugs.com
AUGUST 17, 2023
17, 2023 -- The U.S. Food & Drug Administration has approved Akeega (niraparib and abiraterone acetate) for the treatment of BRCA-positive metastatic castration-resistant prostate cancer. The approval makes Akeega the first and. THURSDAY, Aug.

Drug Discovery World
FEBRUARY 22, 2024
trillion in 2023, according to data analysts GlobalData. The top 20 global biopharmaceutical companies experienced varied year-on-year (YoY) market capitalisation shifts in 2023 amid the macroeconomic headwinds, steep patent cliffs and the commencement of US drug price negotiations under the Inflation Reduction Act (IRA).

Drugs.com
JUNE 26, 2023
MONDAY, June 26, 2023 -- The U.S. Food and Drug Administration has approved Talzenna (talazoparib) with enzalutamide for homologous recombination repair (HRR) gene-mutated metastatic castration-resistant prostate cancer (mCRPC). The approval was.

Drugs.com
JULY 28, 2023
FRIDAY, July 28, 2023 -- The U.S. Food and Drug Administration has approved Xdemvy (lotilaner ophthalmic solution) 0.25 The approval was based on results from two. percent for the treatment of Demodex blepharitis, caused by Demodex mites.

Drugs.com
JUNE 23, 2023
FRIDAY, June 23, 2023 -- The U.S. Food and Drug Administration on Thursday approved the drug Elevidys, the first gene therapy for the treatment of children with Duchenne muscular dystrophy (DMD). The groundbreaking treatment will not be cheap:

Drugs.com
DECEMBER 21, 2023
21, 2023 -- A newly approved test can determine whether a person has a genetically driven risk of becoming addicted to opioids. THURSDAY, Dec. The AvertD test, the first of its kind, uses a DNA sample swabbed from a patient’s cheek to.

Drugs.com
JULY 13, 2023
THURSDAY, July 13, 2023 -- The U.S. Food and Drug Administration on Thursday approved the nation's first over-the-counter birth control pill, a move that will likely pave the way for far greater access to contraception for Americans. Women will be.

Drugs.com
JUNE 23, 2023
FRIDAY, June 23, 2023 -- The U.S. Food and Drug Administration on Thursday approved the drug Elevidys, the first gene therapy for the treatment of children with Duchenne muscular dystrophy (DMD). The groundbreaking treatment will not be cheap:

Drugs.com
AUGUST 3, 2023
3, 2023 -- The U.S. Food and Drug Administration has approved RiVive, an over-the-counter (OTC) naloxone hydrochloride nasal spray for emergency treatment of known or suspected opioid overdose. "We THURSDAY, Aug. We know naloxone is a powerful tool to.

FDA Law Blog: Biosimilars
MARCH 29, 2023
Richardson — Early on March 29, 2023, FDA announced the landmark approval of Narcan (naloxone hydrochloride) Nasal Spray for use as a nonprescription opioid overdose reversal agent. According to this announcement, FDA approval of RiVive is anticipated in July 2023 and the U.S. By Kalie E.

Drug Discovery World
MARCH 18, 2024
The US Food and Drug Administration (FDA) has granted orphan drug and paediatric exclusivity to Cresemba (isavuconazonium sulfate) for the treatment of invasive aspergillosis (IA) and invasive mucormycosis (IM) in paediatric patients.
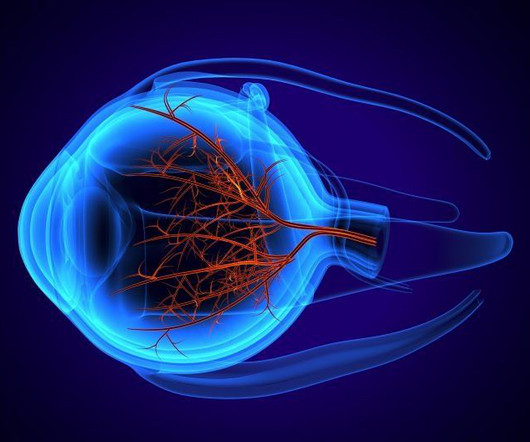
Drugs.com
AUGUST 9, 2023
9, 2023 -- The U.S. Food and Drug Administration has approved Izervay (avacincaptad pegol intravitreal solution) for the treatment of geographic atrophy secondary to age-related macular degeneration. The approval was based on two. WEDNESDAY, Aug.
Drug Discovery World
SEPTEMBER 12, 2024
Only two drugs were approved exclusively for children and young people with cancer by the European Medicines Agency (EMA) and five by the US Food and Drug Administration (FDA) from 2007 to 2022. In contrast, 14 new cancer medicines for adults received FDA approval in 2023 alone.
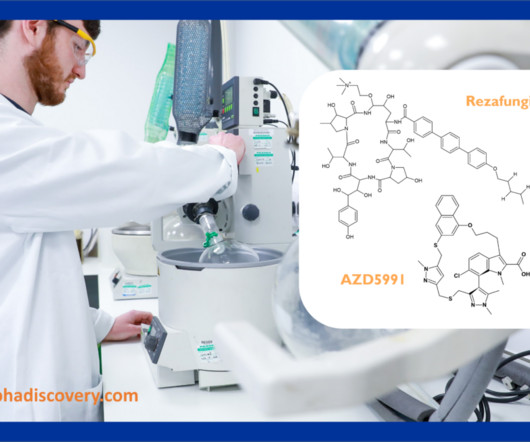
Metabolite Tales Blog
MAY 3, 2023
Hypha’s Q2 2023 Newsletter – acyl glucuronides, hydroxylated metabolites and our latest blogs In our Q2 2023 newsletter we look at the synthesis of a prominent acyl glucuronide of the Mcl-1 inhibitor AZD5991, hydroxylated metabolites of the recently FDA approved rezafungin, as well as links to our latest blogs.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content