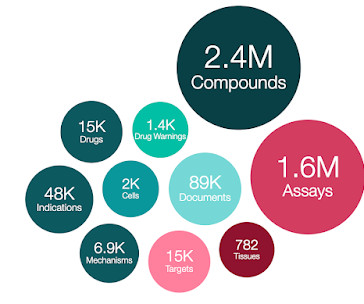
ChEMBL 34 is out!
The ChEMBL-og
APRIL 16, 2024
71 out of the 882 newly added EMA drugs are only authorised by EMA, rather than from other regulatory bodies e.g. FDA. Zimmermann Lab Biotransformation data Dec 2023 (src_id = 69): 271 compounds have been tested for biotransformation in 68 bacterial species and 28 bacterial communities. University of Dundee: T. University of Dundee: T.

























Let's personalize your content