EU Pharmaceutical Legislation Reform: Where Are We Now?
thought leadership
MARCH 26, 2024
On April 26, 2023, the European Commission proposed a new package of pharmaceutical legislation 1 to revise many of the currently applicable Regulations.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
thought leadership
MARCH 26, 2024
On April 26, 2023, the European Commission proposed a new package of pharmaceutical legislation 1 to revise many of the currently applicable Regulations.

Agency IQ
OCTOBER 27, 2023
What we expect European regulators to do in November 2023 In this new recurring feature, AgencyIQ, through public data and previous analysis, determines what European medicine and device regulators will likely do in the month ahead, including key deadlines, meetings, events, planned regulations, comment periods, and more.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Agency IQ
AUGUST 2, 2024
What we expect European regulators to do in August and September 2024 In this recurring feature, AgencyIQ, through public data and previous analysis, determines what European medicine and device regulators will likely do in the month ahead, including key deadlines, meetings, events, planned regulations, comment periods, and more.

Agency IQ
MAY 31, 2024
What we expect European regulators to do in June 2024 In this recurring feature, AgencyIQ, through public data and previous analysis, determines what European medicine and device regulators will likely do in the month ahead, including key deadlines, meetings, events, planned regulations, comment periods, and more.

The Premier Consulting Blog
MAY 4, 2023
billion in 2022 and is expected to grow approximately eight percent from 2023 to 2030 [1]. The FDA has determined that ophthalmic dispensers are now regulated as devices, and the drug and device together are regulated as a combination product. The global ophthalmic drugs market size was valued at $33.81

PPD
OCTOBER 18, 2023
There has been an unprecedented expansion of different types of pharmaceutical therapies, as well as a change in the overall approach to health care. Continued expansion of the biotech industry is occurring in parallel, with an estimated compound annual growth rate of 13.96% from 2023-2030.

Agency IQ
NOVEMBER 20, 2023
BY KIRSTEN MESSMER, PHD, RAC | NOV 13, 2023 8:39 PM CST Background on electronic Product Information The European Commission (EC) has flagged the move to an electronic format for some product information as an area of improvement. readability of package leaflet, patient engagement). readability of package leaflet, patient engagement).

Agency IQ
JULY 8, 2024
What We Expect the FDA to do in July and August 2024 In this ongoing feature, AgencyIQ looks at public data to determine what the FDA is likely to do in the months ahead, including key deadlines, meetings, events, planned regulations, comment periods and more. This is what OIRA is currently reviewing.

FDA Law Blog: Biosimilars
JANUARY 3, 2024
Specifically, CVM ensures that animal drugs are safe and effective, properly made, and adequately labeled and packaged; food-producing animals only take drugs that would be safe for humans to consume; pet foods and additives are safe; and educates the public, monitors the market, and encourages development of new animal health products.

Agency IQ
OCTOBER 6, 2023
BY RAYAN BHARGAVA, MSC | OCT 3, 2023 7:07 PM CDT PFAS background Commonly used in textiles, impregnation agents, paints, firefighting foams (AFFF), and many other applications, per- and polyfluoroalkyl substances (PFAS) have been linked with the incidence of cancer, liver damage, and fertility complications , among other health issues.

Agency IQ
SEPTEMBER 6, 2023
BY ALEXANDER GAFFNEY, MS, RAC SEP 1, 2023 6:21 PM CDT Regulatory background A warning letter is a significant occurrence for a company, and in particular if it follows an inspection of a company’s facility. What should the meeting request package include?

Agency IQ
MAY 3, 2024
What We Expect the FDA to do in May and June 2024 In this ongoing feature, AgencyIQ looks at public data to determine what the FDA is likely to do in the months ahead, including key deadlines, meetings, events, planned regulations, comment periods and more.

Drug Discovery World
MARCH 29, 2023
On January 31 2023, an internally circulated version of the European Commission’s overhaul of the EU’s pharma legislation was leaked, and published by Politico 1. She also does not believe that the problems with Europe’s medicines can be solved with regulation. appeared first on Drug Discovery World (DDW).

Agency IQ
NOVEMBER 3, 2023
BY KIRSTEN MESSMER, PHD, RAC | OCT 27, 2023 8:03 PM CDT Background on E.U. The Pharmaceutical Group of the European Union (PGEU) conducted a survey finding shortages worsened in 2022 compared to 2021, with most European countries reporting worsening. healthcare systems for years. See AgencyIQ’s analysis of EMA’s milestones.]

FDA Law Blog: Biosimilars
AUGUST 17, 2023
Tobolowsky — In January 2023, Vanda Pharmaceuticals, Inc. FDA-2023-P-0313 and FDA-2023-P-0344 ) regarding its product Hetlioz (tasimelteon). FDA regulations, at 21 C.F.R. As such, Vanda’s citizen petition stated that these approvals are “clearly erroneous under the FDCA and agency regulations and will put.

Agency IQ
AUGUST 2, 2024
What We Expect the FDA to do in August and September 2024 In this ongoing feature, AgencyIQ looks at public data to determine what the FDA is likely to do in the months ahead, including key deadlines, meetings, events, planned regulations, comment periods and more.

Agency IQ
JUNE 26, 2023
BY AMANDA CONTI, ALEXANDER GAFFNEY, MS, RAC JUN 21, 2023 6:53 PM CDT Background: legislative efforts to bolster rare disease drug development Defining a “rare disease:” In the U.S., legislators have asked the FDA to study the way that rare disease drugs are regulated in the U.S. regulators. Recently, U.S. In particular, Sec.

Drug Discovery World
OCTOBER 3, 2023
Plans to overhaul the EU regulation on rare disease therapies risk undermining two decades of progress, a new report claims. The assessment, commissioned by the European Federation of Pharmaceutical Industries and Associations (EFPIA), was conducted by Dolon, a strategic consultancy specialising in rare diseases.

Agency IQ
JANUARY 19, 2024
Commission unveils “one substance, one assessment” reform package The Commission has long aspired to realize its one substance, one assessment concept in the EU’s chemical regulatory regime. With the publication of three proposed legal acts, this concept is one step closer to streamlining the way the bloc regulates chemicals.

Agency IQ
JANUARY 12, 2024
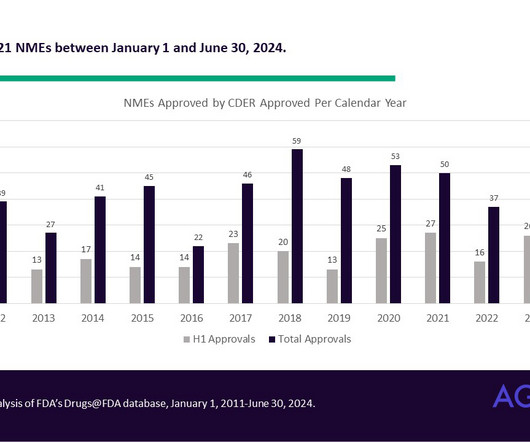
CDER’s latest novel drug approvals report shows how the pandemic is still affecting some drug approvals According to a report released this week describing CDER’s approval of 55 novel drugs in 2023, the agency failed to meet its goal dates under the Prescription Drug User Fee Act (PDUFA) Commitment Letter for 11% of applications – a record high.

Agency IQ
MAY 3, 2024
What we expect European regulators to do in May 2024 In this recurring feature, AgencyIQ, through public data and previous analysis, determines what European medicine and device regulators will likely do in the month ahead, including key deadlines, meetings, events, planned regulations, comment periods, and more.

Agency IQ
DECEMBER 1, 2023
What We Expect the FDA to do in December 2023 In this ongoing feature, AgencyIQ looks at public data to determine what the FDA is likely to do in the month ahead, including key deadlines, meetings, events, planned regulations, comment periods and more.

Agency IQ
JUNE 16, 2023
The 51 regulations that FDA is currently working on The FDA today unveiled its much-anticipated Spring 2023 Unified Agenda, a document outlining the regulations the agency plans to release in 2023 and beyond. The latest update, announced this week, is known as the Spring 2023 Unified Agenda.

Agency IQ
MARCH 15, 2024
California publishes draft regulations for landmark plastic pollution reduction act CalRecycle has launched the formal rulemaking process for Senate Bill 54, which will implement a sweeping plan to reduce plastic waste in the state by 2032. By 2028, the state will require the recycling of 30% of single-use packaging and food service ware.

Agency IQ
JULY 12, 2024
Regulation (EU) 2022/123 , which called for a “reinforced role for EMA in crisis preparedness and management for medicinal products and medical devices,” expanded EMA’s mandate to monitor and mitigate shortages at the E.U. One of the things required by the Commissioner in October 2023 was an analysis of the E.U.

Agency IQ
OCTOBER 27, 2023
What We Expect the FDA to do in November 2023 In this ongoing feature, AgencyIQ looks at public data to determine what the FDA is likely to do in the month ahead, including key deadlines, meetings, events, planned regulations, comment periods and more.

Agency IQ
NOVEMBER 27, 2023
The regulations also provided clarification regarding what does not constitute a “true statement,” including false or misleading information, failure to reveal important facts, and/or the lack of a fair balance between side effects/contraindications and effectiveness.

Agency IQ
NOVEMBER 3, 2023
BY CHELSEY MCINTYRE, PHARMD | OCT 30, 2023 10:57 PM CDT Background: What are microbiome-based therapies? The prebiotic may either feed the probiotic that it is packaged with, or it may feed another beneficial microbe that is intended to complement the activity of the probiotic. The regulation of dietary supplements in the U.S.

Agency IQ
JUNE 21, 2024
Commission proposes guidelines to flesh out the newly finalized Variations Regulation This week, the newly finalized Variations Regulation was published in the Official Journal of the E.U. An updated guideline implementing the changes from the new regulation is now open for public consultation until August 23, 2024.

The Premier Consulting Blog
APRIL 27, 2023
Then, in 1997, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) formed the ICH M8 Expert Working Group/Implementation Working Group (EWG/IWG). As of April 2023, the FDA is overseeing its eCTD v4.0 Implementation Package v1.5 Implementation Package v1.5

Drug Discovery World
AUGUST 10, 2023
In this article, Amplexor asks some of the company’s closest industry partners and BE THE EXPERT event speakers for their views on the biggest developments in the Life Sciences Regulatory sphere over the last 12 months – and explore some of the most prominent themes and priorities seen throughout 2023.

Agency IQ
APRIL 12, 2024
The regulations also explain that this trait can be demonstrated via appropriate laboratory tests or adequately controlled clinical data. Potency assays for cell and gene therapies: The newest guidance At the very end of 2023, the FDA released a draft guidance on establishing CGT product potency to replace its previous 2011 version.

Agency IQ
AUGUST 4, 2023
BY KIRSTEN MESSMER, PHD, RAC AUG 2, 2023 12:18 AM CDT Background The U.K.’s pharmaceutical law ceased to apply to the U.K. s Medicines and Healthcare products Regulatory Agency (MHRA) has been releasing guidance for the pharmaceutical and medical device industry to aid product development and approval after the separation.

Agency IQ
DECEMBER 15, 2023
The 174 regulations the EPA is currently working on The Environmental Protection Agency has unveiled its Fall 2023 Unified Agenda, which provides a look into the agency’s regulatory agenda for the upcoming year. The Fall 2023 edition was released on December 6. BY WALKER LIVINGSTON, ESQ, PATRICIA ISCARO, ESQ. |

Agency IQ
NOVEMBER 27, 2023
BY KIRSTEN MESSMER, PHD, RAC | NOV 17, 2023 4:46 PM CST Quick background on nitrosamines in global medicinal products Nitrosamines are a large and diverse class of chemicals, with more than 300 different structurally distinct species known today. In August 2023, the U.K.’s nitroso-propranolol, nitroso-quinapril, etc.) guidance.]

Agency IQ
JUNE 9, 2023
Just over a month ago, the European Commission released its proposal for the new pharmaceutical directive and regulation. The Orphan Regulation entered into force in January, 2000, outlining requirements for orphan designation and incentives, and establishing the EMA Committee for Orphan Medicinal Products (COMP).

Agency IQ
AUGUST 11, 2023
BY KARI OAKES AUG 7, 2023 10:20 PM CDT Quick background on nitrosamines in pharmaceutical products Nitrosamines are a large and diverse class of chemicals, with more than 300 different structurally distinct species known today. It also focused on impurities found in finished pharmaceutical products.

Agency IQ
JUNE 16, 2023
Highlights of the EPA’s Unified Agenda for Spring 2023 The Office of Management and Budget has released the Unified Agenda for Spring 2023, which outlines the regulations that agencies expect to release in 2023 and 2024. The Fall 2022 edition was released in January 2023.

The Pharma Data
NOVEMBER 2, 2020
To this end, the Board of Directors shall be entitled to make adjustments to meet foreign regulations or market conditions, including resolving on cash or other settlement if deemed favorable for Oncopeptides based on foreign tax regulations. per cent on a fully diluted basis. per cent on a fully diluted basis. CET on 3 November 2020.
Agency IQ
JULY 22, 2024
CDER is the FDA office in charge of reviewing pharmaceuticals and therapeutic biologics. All products are received in one of two forms: A New Drug Application (or NDA, for pharmaceuticals) or a Biologics License Application (BLA, for biologics). For a look at some other trends, see Agency’s Fiscal Year 2023 in Review ].
Codon
FEBRUARY 13, 2024
Fast forward to 2023. Regulators traditionally want to see a single, stable, well-characterized drug before giving the green light for it to be tested in a clinical trial, not dozens of different viruses; let alone ones that are best found in unappealing places like sewage , hospital waste, or bird poop.

Agency IQ
JANUARY 12, 2024
But while the plan may be approved, the decision by FDA still leaves open plenty of questions – and the potential that pharmaceutical trade groups may sue to halt the implementation of the plan. label for that product, including patient labeling such as Medication Guides and patient package inserts.
The Pharma Data
JULY 6, 2021
At its Investor Update, GSK announced a package of new financial outlooks and ambitions. New GSK is expected to have a stronger balance sheet (net debt / adjusted EBITDA of <2x following separation) and a progressive dividend policy starting at 45p per share in 2023. These financial outlooks and ambitions represent a step-change.

Agency IQ
APRIL 12, 2024
The vote is in: The European Parliament has adopted the compromise pharmaceutical legislation This week, the European Parliament voted to adopt the compromise texts of both the revised pharmaceutical directive and regulation presented by Parliament’s health committee in March 2024. has a strong voice in the world. citizens; 2.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content