A therapy candidate for fatal prion diseases turns off disease-causing gene
Broad Institute
JUNE 27, 2024
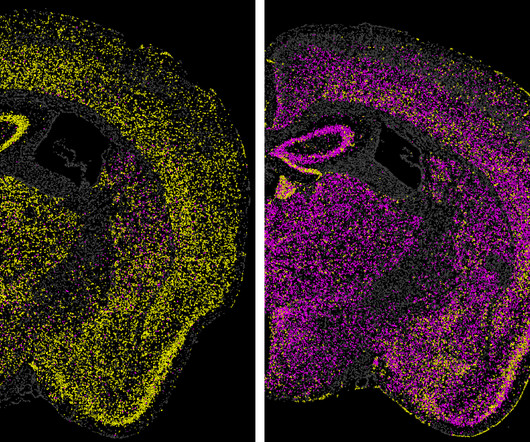
By Greta Friar, Whitehead Institute June 27, 2024 Images of a mouse brain show the effect of a technology called CHARM in turning off the expression of a gene in the brain. The first problem they had to tackle was the editor’s size, because the editor needs to be small enough to be packaged and delivered to specific cells in the body.

















Let's personalize your content