A therapy candidate for fatal prion diseases turns off disease-causing gene
Broad Institute
JUNE 27, 2024
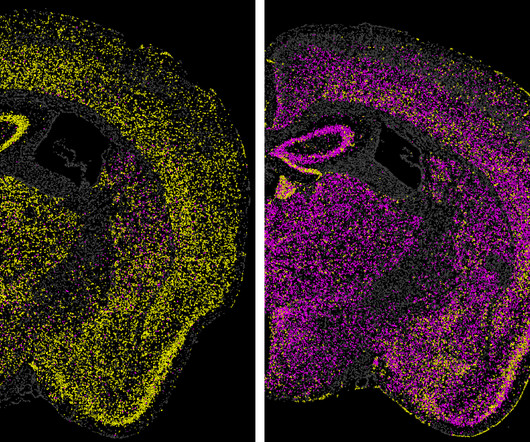
By Greta Friar, Whitehead Institute June 27, 2024 Images of a mouse brain show the effect of a technology called CHARM in turning off the expression of a gene in the brain. Therapy search Vallabh, along with her husband Eric Minikel, leads a lab at Broad focused on developing drugs to prevent and treat prion diseases.










Let's personalize your content