A therapy candidate for fatal prion diseases turns off disease-causing gene
Broad Institute
JUNE 27, 2024
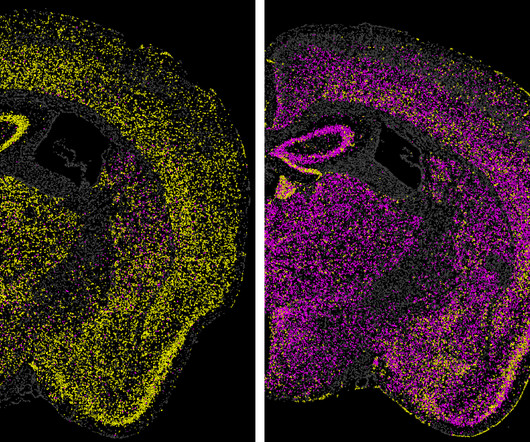
By Greta Friar, Whitehead Institute June 27, 2024 Images of a mouse brain show the effect of a technology called CHARM in turning off the expression of a gene in the brain. Previous research has shown that as little as 21 percent elimination of the protein can improve symptoms. Credit: Neumann EN, Bertozzi TM, et al.





















Let's personalize your content